Draw A Lewis Structure For Hcn
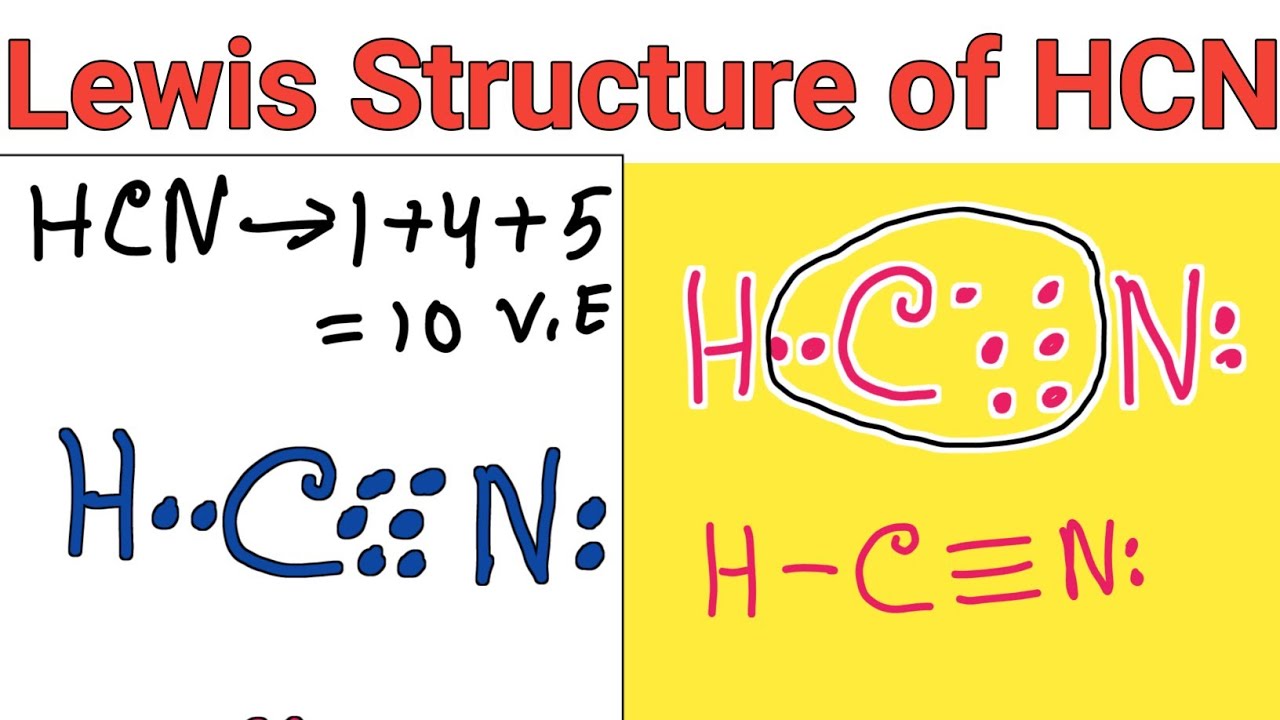
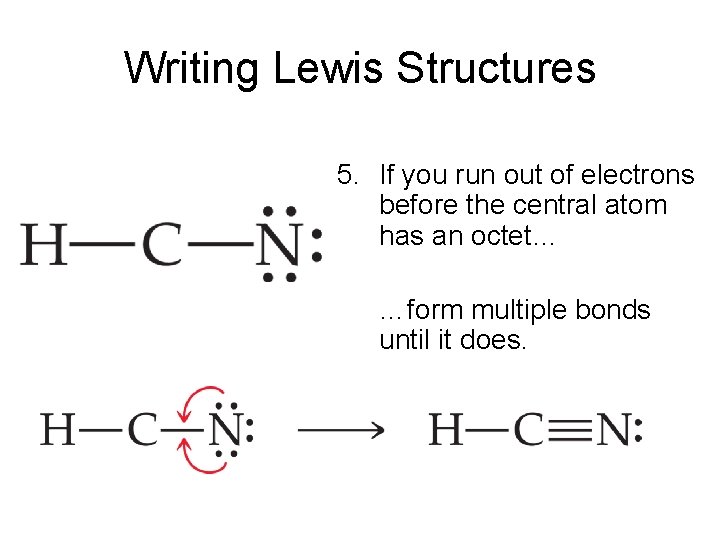
Draw A Lewis Structure For Hcn - Please check your connection and try again. Web hey guys!in this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to. Put least electronegative atom in centre3. #2 mark lone pairs on the atoms. Web definition of valence bond theory. Use these steps to correctly draw the hcn lewis structure: Web first of all, to remind you lewis’s structure is a pictorial representation of different bonds and lone pair of electrons between two or more atoms of a compound. In order to draw the lewis structure of hcn, first of all you have to find the total number. With the lewis structure for hcn you’ll need to. The hydrogen atom is bonded. Web steps of drawing hcn lewis structure. #3 calculate and mark formal charges on the. Here, the given molecule is hcn. Web the lewis structure (lewis dot diagram) for hcn.1. For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Count the valence electrons you can use. Web drawing the lewis structure for hcn. The molecule is made up of one hydrogen ato. Describe the interactions between atoms using lewis. #3 calculate and mark formal charges on the. Web the lewis structure of hcn shows that the carbon atom is the central atom and is covalently bonded to both hydrogen and nitrogen atoms. You'll get a detailed solution. The molecule is made up of one hydrogen ato. In order to find the total valence electrons in hcn molecule, first of. The following procedure will give you the correct lewis structure for any molecule or. We're unable to load study guides on this page. Here, the given molecule is hcn. #3 calculate and mark formal charges on the. 4k views 6 years ago chem 101: Web definition of valence bond theory. The molecule is made up of one hydrogen ato. Establish a general procedure for drawing lewis structures. Put least electronegative atom in centre3. Solution hcn is a linear molecule, since it has only two electron groups and. Describe the interactions between atoms using lewis. This problem has been solved! The molecule is made up of one hydrogen ato. Put the least electronegative atom c in the middle with h and cl on either side. Web hey guys!in this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. Web the lewis structure of hcn shows that the carbon atom is the central atom and is covalently bonded to both hydrogen and nitrogen atoms. How to draw lewis structure for hcn? Put one electron pair in each bond4. In order to find the total valence electrons in hcn molecule, first of all you. For the hcn lewis structure, calculate. Calculate the total number of valence electrons. In order to draw the lewis structure of hcn, first of all you have to find the total number. In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to. 4k views 6 years ago chem 101: In order. Find the total valence electrons in hcn molecule. For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. In order to find the total valence electrons in hcn molecule, first of all you. Web the lewis structure of hcn shows that the carbon atom is the central atom and is covalently bonded to both. Put one electron pair in each bond4. With the lewis structure for hcn you’ll need to. #2 mark lone pairs on the atoms. Step method to draw the lewis structure of hcn. For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Web definition of valence bond theory. This problem has been solved! Put one electron pair in each bond4. Web steps of drawing hcn lewis structure. Make sure you put the correct atom at the center of the hcn molecule. Web first of all, to remind you lewis’s structure is a pictorial representation of different bonds and lone pair of electrons between two or more atoms of a compound. #2 mark lone pairs on the atoms. #1 first draw a rough sketch. Does this molecule exhibit resonance? 1.1k views 3 years ago chemistry. Put least electronegative atom in centre3. Put the least electronegative atom c in the middle with h and cl on either side. Describe the interactions between atoms using lewis. Web hey guys!in this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. 4k views 6 years ago chem 101: Find the total valence electrons in hcn molecule.
HCN Lewis Structure (Hydrogen Cyanide) YouTube

How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN

HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw

Hcn Lewis Structure Bonds Draw Easy

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and

Lewis Diagram For Hcn
Lewis structure of HCN (Hydrogen cyanide) YouTube
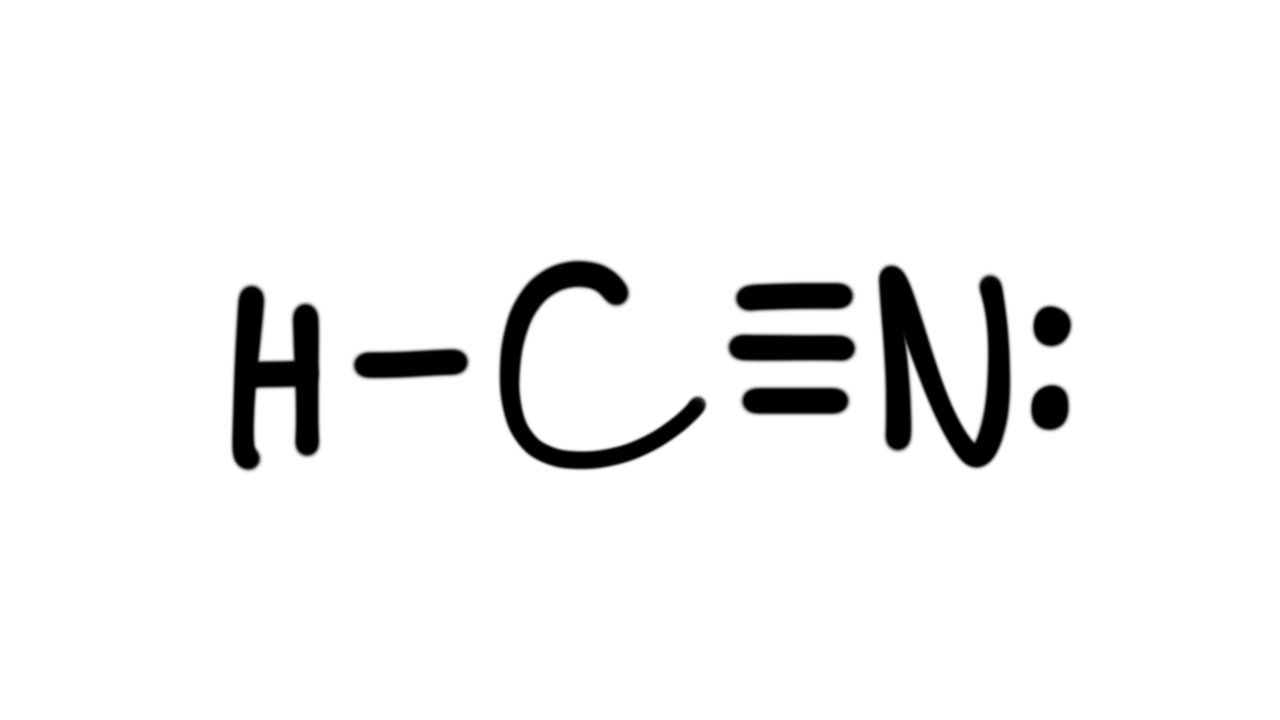
Estrutura De Lewis Hcn
Hcn Lewis Structure Bonds Draw Easy

HCN Lewis Structure How to Draw the Dot Structure II lSCIENCE ll
Solution Hcn Is A Linear Molecule, Since It Has Only Two Electron Groups And.
Web Learn To Draw The Lewis Structure Of Hcn & Understand Molecular Geometry, Shape, & Polarity About The Same By Reading This Article.
#3 Calculate And Mark Formal Charges On The.
In Order To Find The Total Valence Electrons In Hcn Molecule, First Of All You.
Related Post: