Draw An Energy Diagram For An Exothermic Reaction
Draw An Energy Diagram For An Exothermic Reaction - The reactants start with a higher energy level and release energy as they transform into products. Draw the transition state of a reaction. Strong bonds have lower potential energy than weak bonds. The figure below shows basic potential energy diagrams for an endothermic (a) and an exothermic (b) reaction. Exothermic reactions release energy during the reaction. (b) a slow exergonic reaction (high δg ‡, negative δg°); The difference in energy between the reactants and products is the amount of heat energy transferred to the surroundings. (a) a fast exergonic reaction (low δg ‡, negative δg°); The products have a lower energy than the reactants, and so energy is released when the reaction happens. Web this diagram shows that, overall, the reaction is exothermic. Draw the curve in the energy level diagram clearly showing the transition state. A second model for a nucleophilic substitution reaction is called the ' dissociative', or ' sn1' mechanism: The figure below shows basic potential energy diagrams for an endothermic (a) and an exothermic (b) reaction. Web the energy diagram for an exothermic reaction typically shows a decrease in. Web a physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. Ch4 (g) + 2o2 (g) → co2 (g) + 2h2o (l) step 2: (a) a fast exergonic reaction (low δg ‡, negative δg°); It also shows the effect. Draw the curve in the energy level diagram clearly showing the transition state. The chemical equation for the complete combustion of methane is: This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. , and the difference in energy between them. The figure below shows basic potential energy diagrams for an endothermic (a) and an exothermic. The energy released is represented by a negative number on the diagram. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. Draw reaction energy diagrams from the thermodynamic and kinetic data/information. (c) a fast. Strong bonds have lower potential energy than weak bonds. Web in this video, i go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. Web this chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams.. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This problem has been solved! Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. It shows the energy in the. Use a reaction energy diagram to discuss transition states, ea, intermediates &. Ch4 (g) + 2o2 (g) → co2 (g) + 2h2o (l) step 2: You can start with a generic potential energy diagram for an exothermic reaction. Strong bonds have lower potential energy than weak bonds. Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. The products will end. Web how to draw energy profile diagram and energy level diagram of exothermic and endothermic reaction. Use a reaction energy diagram to discuss transition states, ea, intermediates & rate determining step. You can start with a generic potential energy diagram for an exothermic reaction. Draw the curve in the energy level diagram clearly showing the transition state. A second model. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Combustion reactions are always exothermic (δ h is negative) so the reactants should be drawn higher in energy than the products. Web the energy diagram for an exothermic reaction typically shows a decrease in potential energy as the reactants are converted to products. Strong. Web this diagram shows that, overall, the reaction is exothermic. Web the typical energy level diagram for an exothermic reaction is shown below: The figure below shows basic potential energy diagrams for an endothermic (a) and an exothermic (b) reaction. Web a physical or chemical process can be represented using an energy diagram, which shows how the potential energy of. Draw reaction energy diagrams from the thermodynamic and kinetic data/information. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. Strong bonds have lower potential energy than weak bonds. Exothermic reactions release energy during the reaction. In endothermic reactions, the reactants have stronger bonds than the products. Ch4 (g) + 2o2 (g) → co2 (g) + 2h2o (l) step 2: Web the energy diagram for an exothermic reaction typically shows a decrease in potential energy as the reactants are converted to products. Web the typical energy level diagram for an exothermic reaction is shown below: The products will end at a higher energy than the reactants? Draw the curve in the energy level diagram clearly showing the transition state. A second model for a nucleophilic substitution reaction is called the ' dissociative', or ' sn1' mechanism: It also shows that the molecules have to possess enough energy (called activation energy) to get the reactants over what we think of as the activation energy barrier. Use a reaction energy diagram to discuss transition states, ea, intermediates & rate determining step. Label the parts that represent the reactants, products, transition state, activation energy, and heat of reaction. The chemical equation for the complete combustion of methane is: In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products.
Energy level diagrams Endothermic & Exothermic reactions
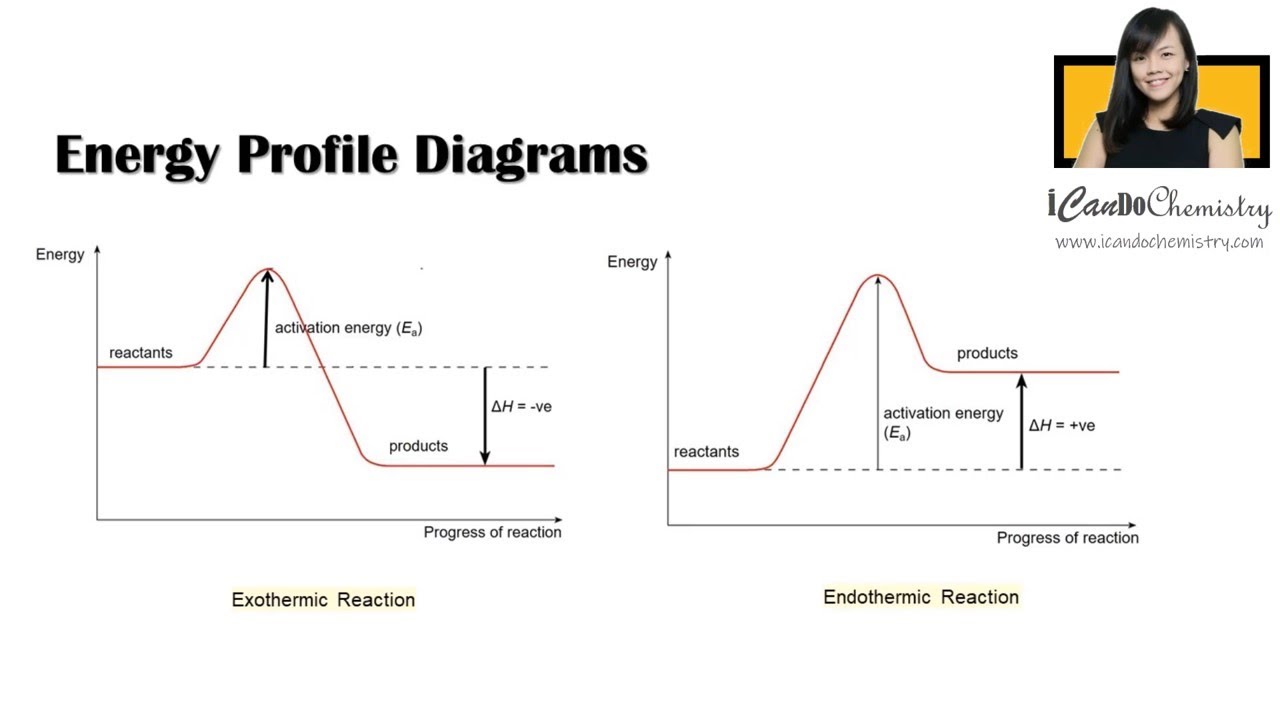
How to draw Energy Profile Diagram and Energy Level Diagram of

Energy Diagram — Overview & Parts Expii
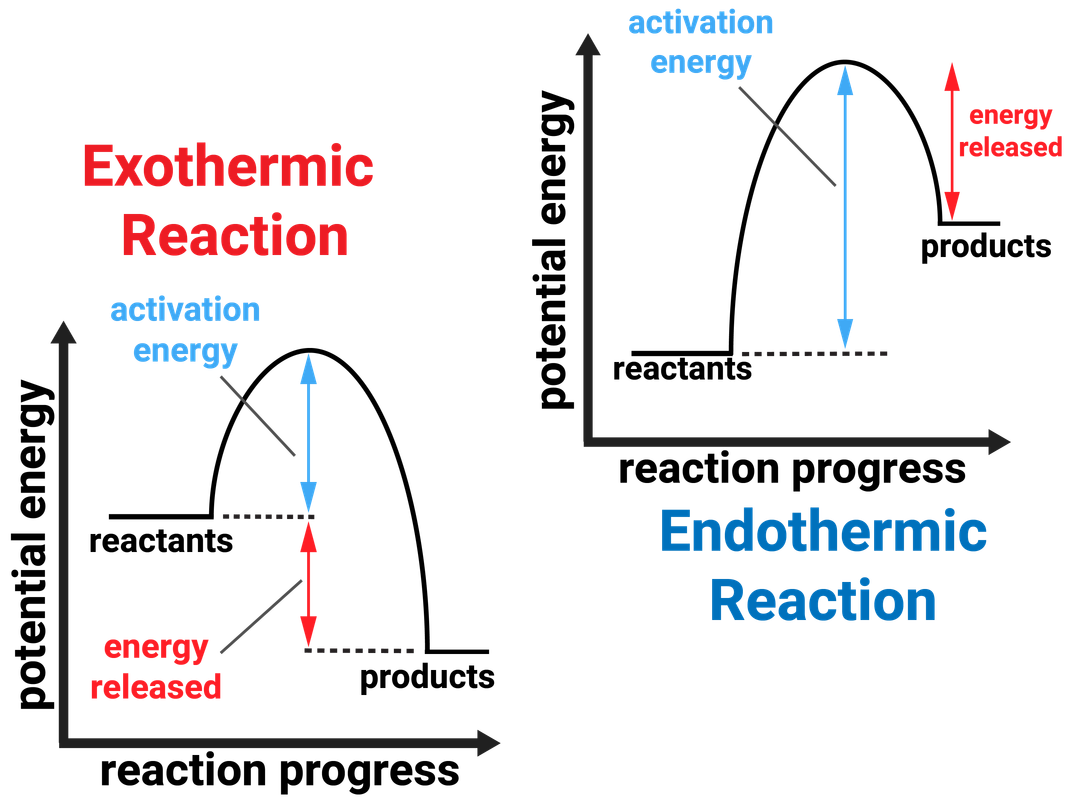
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk
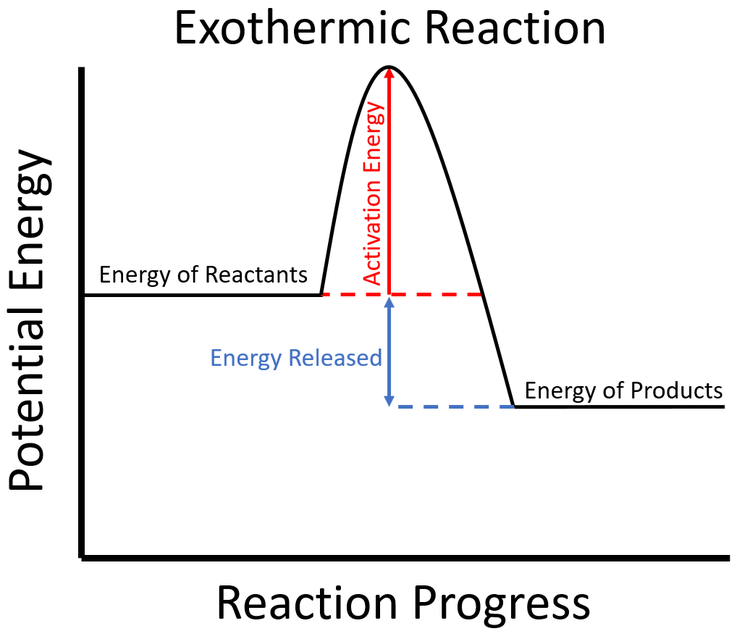
Exothermic Key Stage Wiki
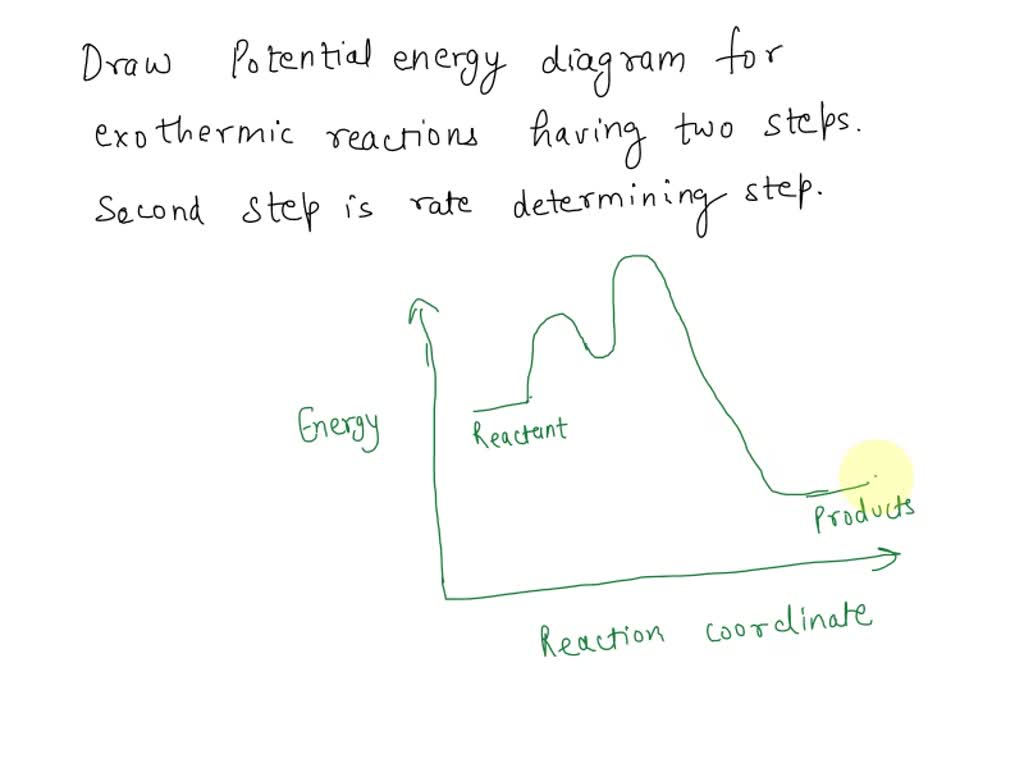
SOLVED Draw the energy diagram for a twostep exothermic reaction
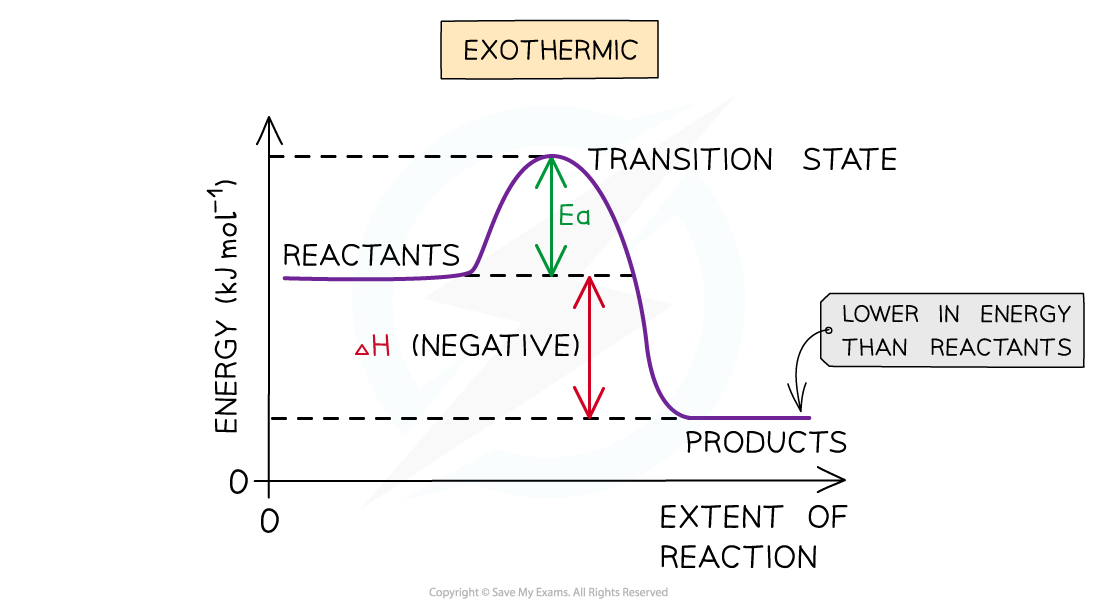
CIE A Level Chemistry复习笔记1.5.2 Energy Level Diagrams翰林国际教育
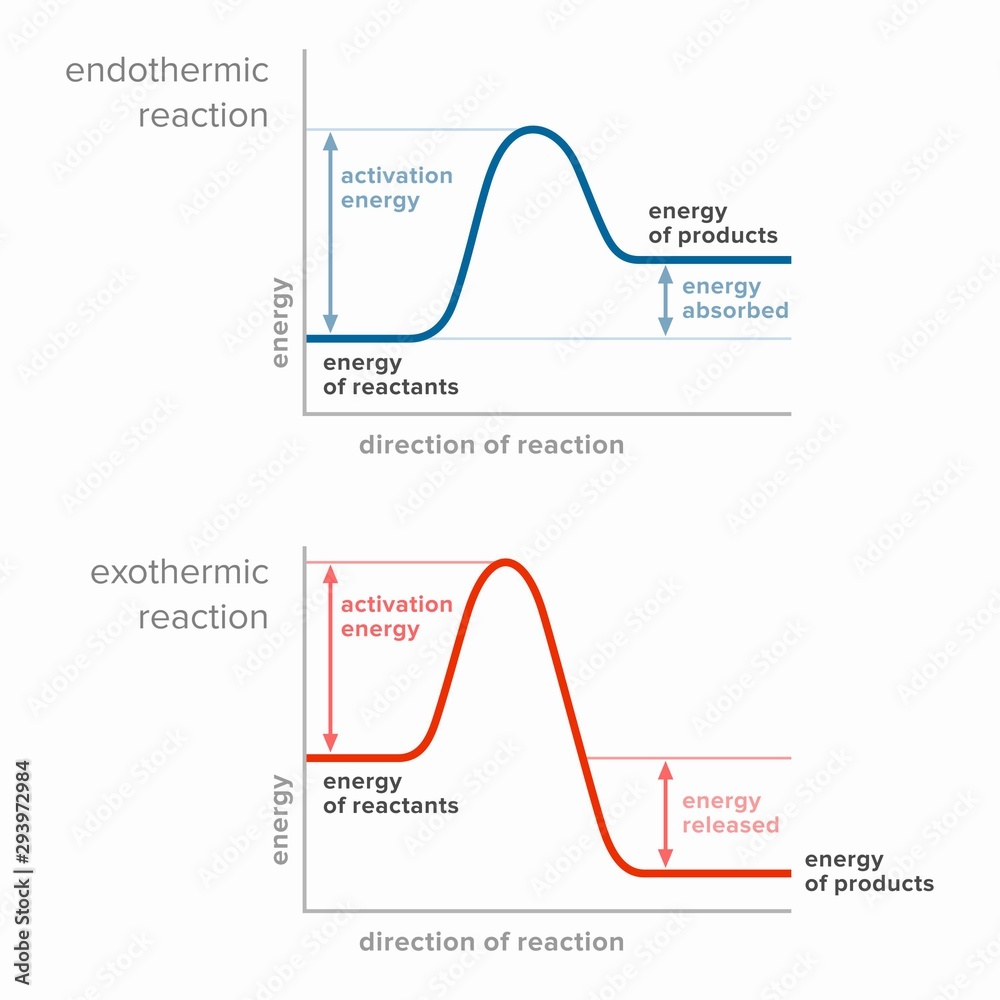
Activation energy in endothermic and exothermic reactions. Stock

Exothermic Reaction Energy Graph
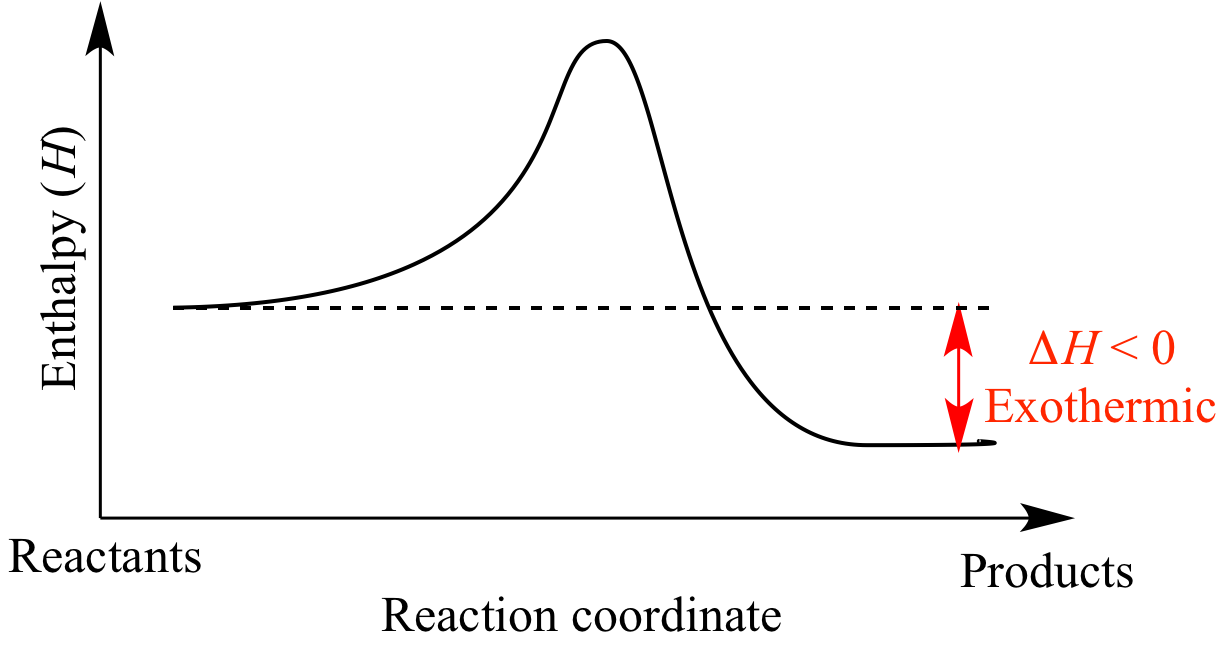
Illustrated Glossary of Organic Chemistry Exothermic
The Products Have A Lower Energy Than The Reactants, And So Energy Is Released When The Reaction Happens.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
The Figure Below Shows Basic Potential Energy Diagrams For An Endothermic (A) And An Exothermic (B) Reaction.
Web A Physical Or Chemical Process Can Be Represented Using An Energy Diagram, Which Shows How The Potential Energy Of The Initial State Relates To The Potential Energy Of The Final State.
Related Post: