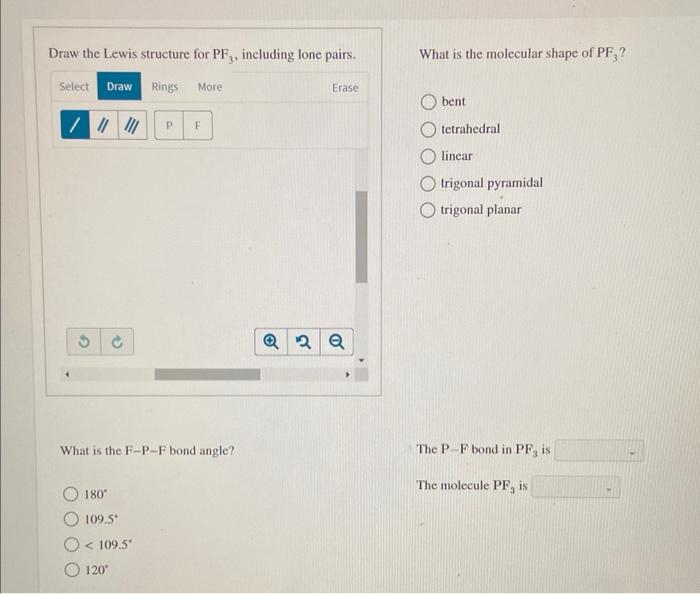
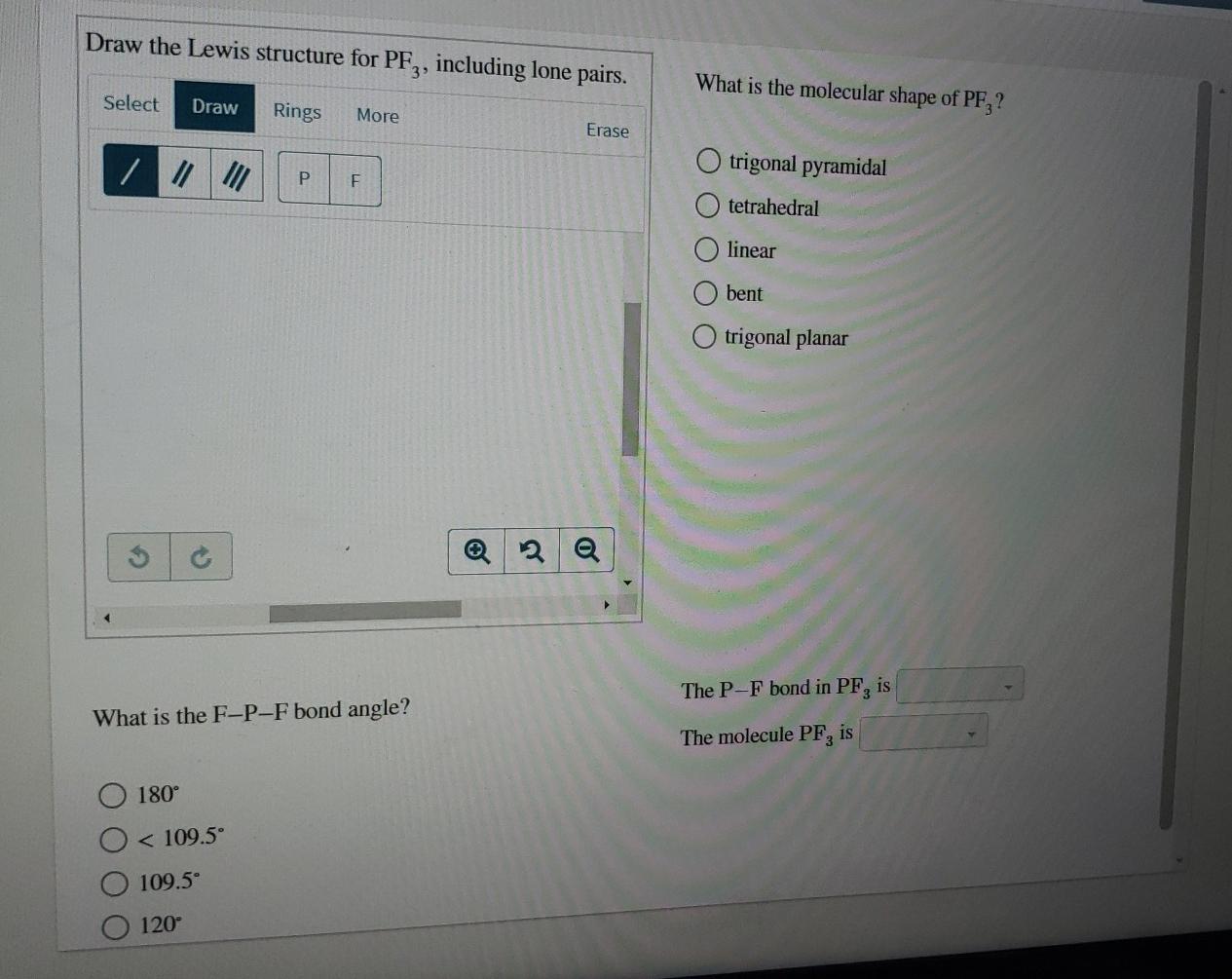
Draw The Lewis Structure For Pf3 Including Lone Pairs
Draw The Lewis Structure For Pf3 Including Lone Pairs - Draw a lewis structure for each of the following compounds. What is the molecular shape of pf,? In order to draw the lewis structure of pf3, first. By using the following steps, you can easily draw the lewis structure of pf 3: Web if you can do those lewis structures pf 3 will be easy. Web in the pf 3 molecule, there will be a lone pair over phosphorus present in sp 3 hybridized orbital and three fluorine have three pairs of lone pair. Draw the lewis structure for pf,, including lone pairs. Calculate the total number of valence electrons. Select draw rings more erase / o trigonal pyramidal р f. Send feedback | visit wolfram|alpha. Bent tetrahedral linear trigonal pyramidal trigonal planar what is the. Web draw lewis structures depicting the bonding in simple molecules. What is the molecular shape of pf3 ? Back bonding occurs between the atoms, where one has a lone pair of electrons while the other has a vacant orbital. Calculate the total number of valence electrons. Web if you can do those lewis structures pf 3 will be easy. Web in the pf 3 lewis structure, there are three single bonds around the phosphorus atom, with three fluorine atoms attached to it. Web in the pf 3 molecule, there will be a lone pair over phosphorus present in sp 3 hybridized orbital and three fluorine have. Bent tetrahedral linear trigonal pyramidal trigonal planar what is the. Web we show you how to draw the lewis structure and determine the moleculargeometry for phosphorus trifluoride (pf3). Web added jun 9, 2014 by webtester in chemistry. Send feedback | visit wolfram|alpha. Understand the proper use of the octet rule to predict bonding in simple molecules. Here, the given molecule is pf3 (phosphorus trifluoride). Web in the pf 3 molecule, there will be a lone pair over phosphorus present in sp 3 hybridized orbital and three fluorine have three pairs of lone pair. Web draw lewis structures depicting the bonding in simple molecules. Draw the lewis dot structure for pf3. Calculate the total number of valence. Draw the lewis structure for pf3, including lone pairs. Draw a lewis structure for each of the following compounds. Include all lone pair electrons. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. 91k views 10 years ago. The three f atoms bond to the p, but this leaves a lone pair as the fourth electron pair in the valence shell of. In the pf 3 lewis structure phosphorus (p) is the least electronegative so it goes in the center. By using the following steps, you can easily draw the lewis structure of pf 3: Web draw lewis. Web draw lewis structures depicting the bonding in simple molecules. Here, the given molecule is pf3 (phosphorus trifluoride). Understand the proper use of the octet rule to predict bonding in simple molecules. Draw the lewis structure for pf3, including lone pairs. In the pf 3 lewis structure phosphorus (p) is the least electronegative so it goes in the center. Web draw lewis structures depicting the bonding in simple molecules. What is the molecular shape of pf3 ? Web we show you how to draw the lewis structure and determine the moleculargeometry for phosphorus trifluoride (pf3). Include all lone pair electrons. Draw a lewis structure for each of the following compounds. Send feedback | visit wolfram|alpha. Web in the pf 3 molecule, there will be a lone pair over phosphorus present in sp 3 hybridized orbital and three fluorine have three pairs of lone pair. If you realize, a single lone pair of electrons in. Calculate the total number of valence electrons. This widget gets the lewis structure of chemical compounds. What is the molecular shape of pf3 ? Draw the lewis structure for pf,, including lone pairs. What is the molecular shape of pf3 ? Write a lewis structure for the phosphorus trifluoride molecule, pf3. Understand the proper use of the octet rule to predict bonding in simple molecules. Draw a lewis structure for each of the following compounds. Draw a lewis structure for each of the following compounds. Draw the lewis structure for pf,, including lone pairs. Include all lone pair electrons. Web added jun 9, 2014 by webtester in chemistry. Include all lone pair electrons. Write a lewis structure for the phosphorus trifluoride molecule, pf3. Web in the pf 3 lewis structure, there are three single bonds around the phosphorus atom, with three fluorine atoms attached to it. This widget gets the lewis structure of chemical compounds. Web in the pf 3 molecule, there will be a lone pair over phosphorus present in sp 3 hybridized orbital and three fluorine have three pairs of lone pair. Each fluorine atom has three. Calculate the total number of valence electrons. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. In the pf 3 lewis structure phosphorus (p) is the least electronegative so it goes in the center. If you realize, a single lone pair of electrons in. Web if you can do those lewis structures pf 3 will be easy.Solved Draw the Lewis structure for PF3, including lone
SOLVED(a) Draw the dominant Lewis structure for the phosphorus

PF3 Molecular Geometry Science Education and Tutorials
draw the lewis structure for pf3 including lone pairs bettinaniedermaier

draw the lewis structure for pf3 including lone pairs 160vanbruntstreet
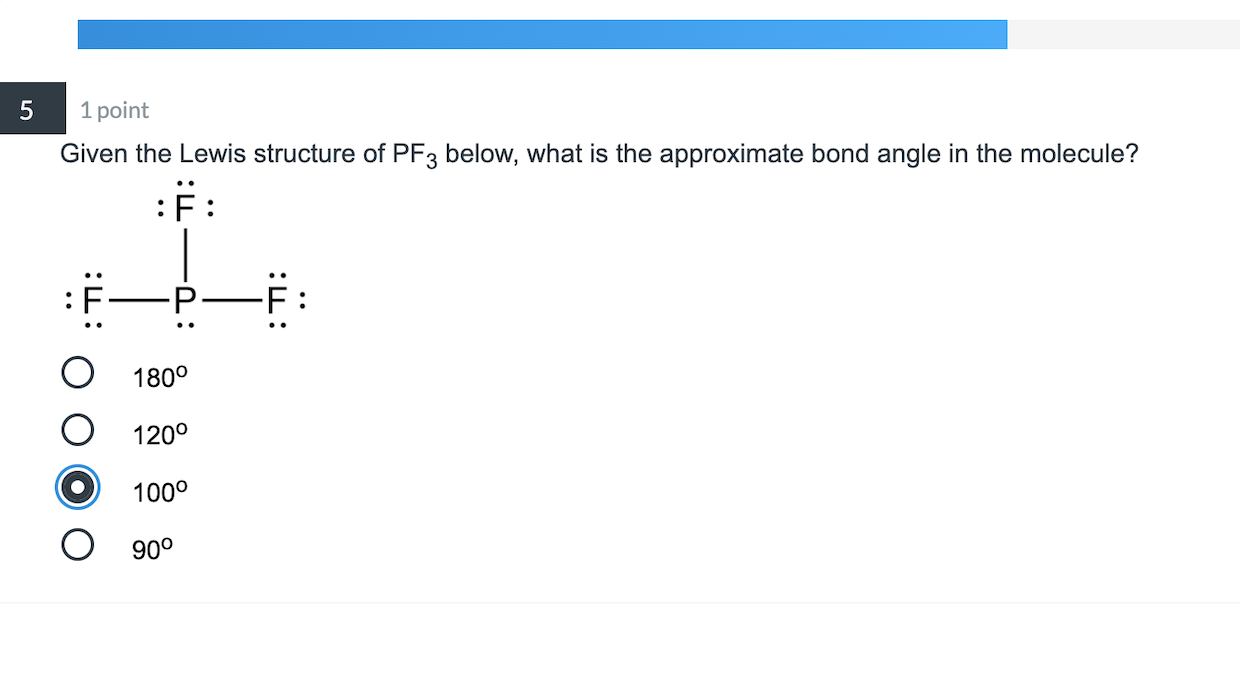
Solved 5 1 point Given the Lewis structure of PF3 below,

PF3 Electron Geometry (Phosphorus trifluoride) YouTube

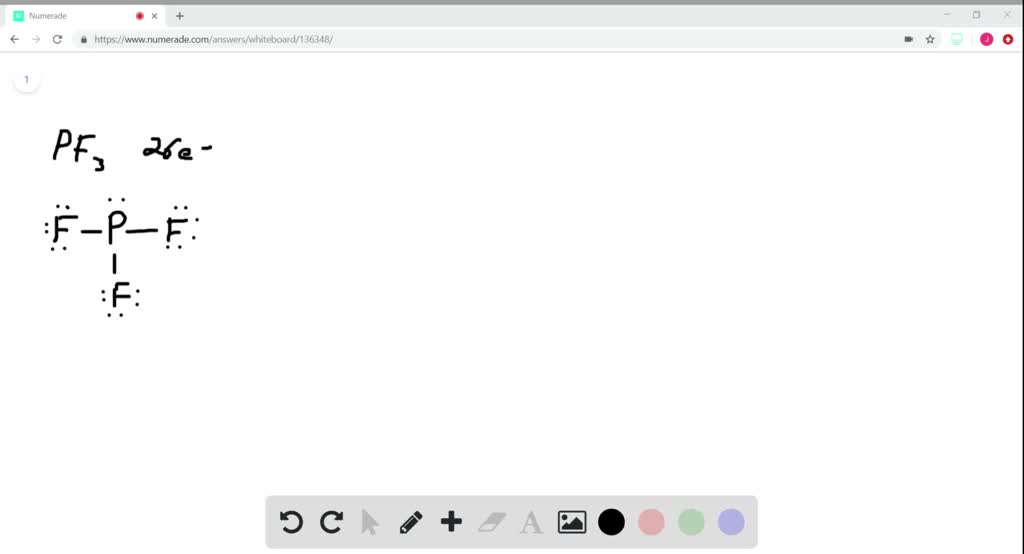
So far, we’ve used 26 of the PF3 Lewis structure’s total 26 outermost

Pf3 Lewis Structure Molecular Geometry

The first step is to put five valence electrons around the phosphorus
Draw The Lewis Structure For Pf3, Including Lone Pairs.
Understand The Proper Use Of The Octet Rule To Predict Bonding In Simple Molecules.
In Order To Draw The Lewis Structure Of Pf3, First.
What Is The Molecular Shape Of Pf,?
Related Post: