Draw The Lewis Structure For Sf4
Draw The Lewis Structure For Sf4 - Therefore sulfur becomes the center. In this structure sulfur will have ten valence electrons. Web to draw the sf4 lewis structure, follow these steps: Sulfur has a valence of 6. Web 5 steps to draw the lewis structure of sf4 step #1: 100% (2 ratings) share share. Here, the given molecule is sf4. First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the. Calculate the total number of valence electrons. Here’s how to approach this question. Sulfur is in group 16 of the periodic table, so it has 6. #2 mention lone pairs on the atoms. What is the hybridization and formal charge on the sulfur? Web for sf4 draw an appropriate lewis structure. #1 draw a rough skeleton structure. First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the. Web 5 steps to draw the lewis structure of sf4 step #1: Web for sf4 draw an appropriate lewis structure. Here’s how to approach this question. Web which of the following compounds is sulfur tetrafluoride? Web which of the following compounds is sulfur tetrafluoride? Web here’s how you can easily draw the sf 4 lewis structure step by step: Calculate the total number of valence electrons. Here’s the best way to solve it. In order to draw the lewis. #2 mention lone pairs on the atoms. 2.8k views 11 years ago chemistry lewis dot structures. Therefore sulfur becomes the center. Web to draw the sf4 lewis structure, follow these steps: 100% (2 ratings) share share. 100% (2 ratings) share share. Sulfur is in group 16 of the periodic table, so it has 6. Sf 4 is lewis structure with sulfur (s). Determine the total number of valence electrons by adding up the valence electrons of all atoms in the molecule. Draw the lewis structure for sf4. 2.8k views 11 years ago chemistry lewis dot structures. Sulfur has a valence of 6. Web here’s how you can easily draw the sf 4 lewis structure step by step: Web in sf4 lewis structure, each fluorine atom has joint with center sulfur atom. #1 draw a rough skeleton structure. Draw the lewis structure for sf4 in the window below and then answer the questions that follow. Web to draw the sf4 lewis structure, follow these steps: Web here’s how you can easily draw the sf 4 lewis structure step by step: Web learn the steps to draw the lewis structure of sf4 in just 1 minute.📌you can draw any. What is the hybridization and formal charge on the sulfur? #1 draw a rough skeleton structure. Web to draw the lewis structure for sf4, we start by determining the total number of valence electrons. Also, there is a lone pair on sulfur atom. 2.8k views 11 years ago chemistry lewis dot structures. Here, the given molecule is sf4. Web which of the following compounds is sulfur tetrafluoride? In order to draw the lewis. First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the. In this structure sulfur will have ten valence electrons. 100% (2 ratings) share share. Web for sf4 draw an appropriate lewis structure. Here’s the best way to solve it. #2 mention lone pairs on the atoms. Therefore sulfur becomes the center. Web learn the steps to draw the lewis structure of sf4 in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in this artic. Web here’s how you can easily draw the sf 4 lewis structure step by step: #2 mention lone pairs on the atoms. Here’s the best way to solve it. What is the hybridization and formal charge on the sulfur? Draw the lewis structure for sf4. 2.8k views 11 years ago chemistry lewis dot structures. Sf 4 is lewis structure with sulfur (s). Here’s how to approach this question. Web to draw the lewis structure for sf4, we start by determining the total number of valence electrons. Web which of the following compounds is sulfur tetrafluoride? Therefore sulfur becomes the center. Sulfur is in group 16 of the periodic table, so it has 6. In this structure sulfur will have ten valence electrons. Draw the lewis structure for sf4 in the window below and then answer the questions that follow. Web we draw lewis structures to predict:
SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
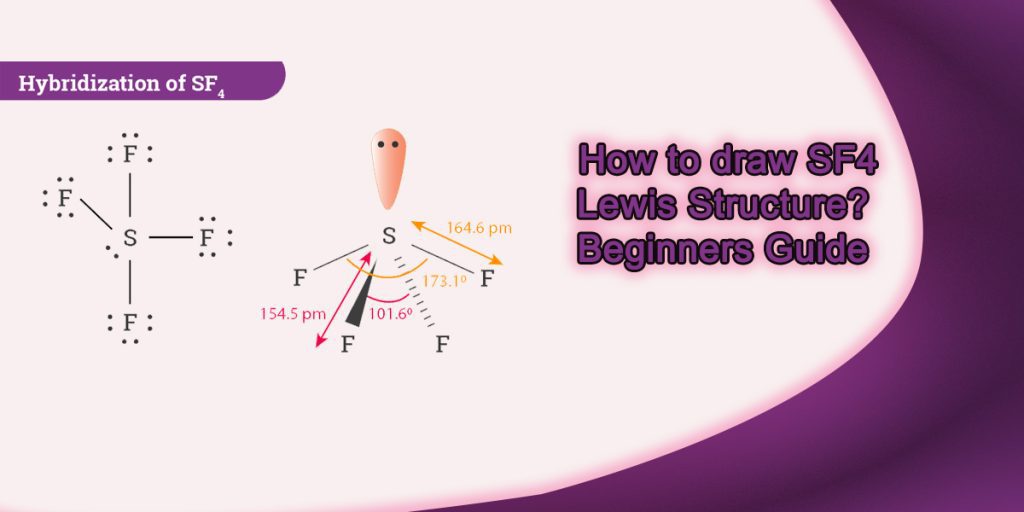
How to draw Sf4 Lewis Structure? Beginners Guide

How to draw Sf4 Lewis Structure? Beginners Guide

How to draw SF4 Lewis Structure? Science Education and Tutorials

SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube

SF4 Lewis Dot structureHow to draw the Lewis structure for SF4. neet

SF4 Lewis Structure ,Valence Electrons,Formal Charge,Octet Rule

How to draw SF4 Lewis Structure? Science Education and Tutorials
Remember That Sulfur Can Hold More Than 8 Valence Electrons.
First, We Arrange The Atoms, Then Distribute The Valence Electrons, And Finally, Place Them Around The.
In Order To Draw The Lewis.
Calculate The Total Number Of Valence Electrons.
Related Post: