Draw The Lewis Structure For The Chlorine Pentafluoride Molecule
Draw The Lewis Structure For The Chlorine Pentafluoride Molecule - #1 first draw a rough sketch. Complete chemical reactions are:cl 2 + h 2 o →. If an overall dipole moment is present, draw the arrow.please. This problem has been solved! Identify both its electron pair and molecular geometries. Web here’s how to approach this question. An explanation of the molecular geometry for the clf5 ion (chlorine pentafluoride) including a description of the clf5 bond angles. Web learn about the formula and structure of chlorine pentafluoride, a molecular compound with five fluorine atoms bonded to a central chlorine atom. #1 draw a rough skeleton structure. A lewis structure is a way to show how. Identify both its electron pair and molecular geometries. If an overall dipole moment is present, draw the arrow.please. Question 4 15 pts draw the lewis structure of chlorine pentafluoride. An explanation of the molecular geometry for the clf5 ion (chlorine pentafluoride) including a description of the clf5 bond angles. Web to draw lewis structures for molecules and polyatomic ions with. Web draw the lewis structure of chlorine pentafluoride. #2 mark lone pairs on the atoms. Find the total valence electrons in clf5 molecule. Identify both its electron pair and molecular geometries. Web here’s how to approach this question. #1 first draw a rough sketch. Draw the lewis structure for the chlorine pentafluoride (cif:) molecule. Web a video explanation of how to draw the lewis dot structure for chlorine pentafluoride, along with information about the compound including formal charges, po. Find the total valence electrons in clf5 molecule. Begin by determining the total number of valence electrons in clf3. #1 first draw a rough sketch. #2 mention lone pairs on the atoms. Web a video explanation of how to draw the lewis dot structure for chlorine pentafluoride, along with information about the compound including formal charges, po. This problem has been solved! Ć с so c e х $ explanation check ar c 30 f3 q 76 f1. Steps of drawing clf5 lewis structure. Count the total number of valence electrons for chlorine and fluorine to use in the lewis structure. Ć с so c e х $ explanation check ar c 30 f3 q 76 f1. Is that molecule a lewis base? Find the total valence electrons in clf5 molecule. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Find the total valence electrons for clf3. Here’s how you can easily draw the clf 5 lewis structure step by step: Count the total number of valence electrons for chlorine and fluorine to use in the lewis structure. #1 draw a rough skeleton structure. Web use these steps to correctly draw the sf 5 cl lewis structure: If an overall dipole moment is present, draw the arrow.please. #3 calculate and mark formal. Web a video explanation of how to draw the lewis dot structure for chlorine pentafluoride, along with information about the compound including formal charges, po. A lewis structure is a way to. Complete chemical reactions are:cl 2 + h 2 o →. Web with what neutral molecule is clo − isoelectronic? Explore the 3d model and. A lewis structure is a way to show how. #2 mark lone pairs on the atoms. If an overall dipole moment is present, draw the arrow.please. Question 4 15 pts draw the lewis structure of chlorine pentafluoride. Explore the 3d model and. Web draw the lewis structure for the chlorine pentafluoride (clf5) molecule. Begin by determining the total number of valence electrons in clf3. Web draw the lewis structure for the chlorine pentafluoride (clf5) molecule. #2 mark lone pairs on the atoms. In order to find the total valence electrons in a clf5. #3 calculate and mark formal. An explanation of the molecular geometry for the clf5 ion (chlorine pentafluoride) including a description of the clf5 bond angles. #2 mark lone pairs on the atoms. Web draw the lewis structure of chlorine pentafluoride. Here’s how you can easily draw the clf 5 lewis structure step by step: Web a video explanation of how to draw the lewis dot structure for chlorine pentafluoride, along with information about the compound including formal charges, po. Draw the lewis structure for the chlorine pentafluoride (cif:) molecule. A lewis structure is a way to show how. Identify both its electron pair and molecular geometries. An explanation of the molecular geometry for the clf5 ion (chlorine pentafluoride) including a description of the clf5 bond angles. 4.8k views 1 year ago. Begin by determining the total number of valence electrons in clf3. Ć с so c e х $ explanation check ar c 30 f3 q 76 f1. Question 4 15 pts draw the lewis structure of chlorine pentafluoride. Explore the 3d model and. #1 first draw a rough sketch. You'll get a detailed solution from a subject matter expert that helps. Complete chemical reactions are:cl 2 + h 2 o →.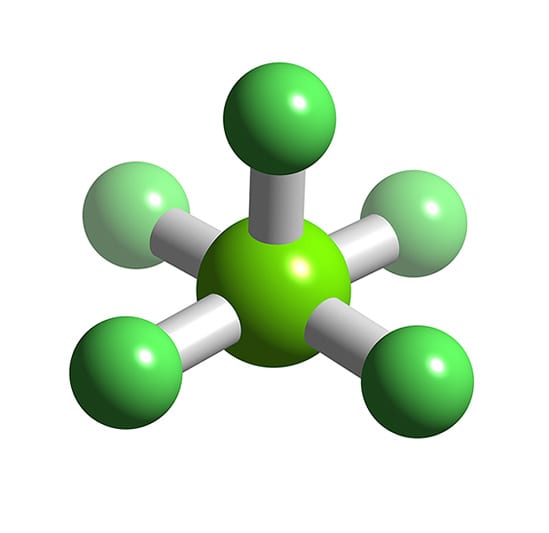
ClF5 Chlorine pentafluoride

ClF5 Lewis Structure How to Draw the Lewis Structure for ClF5

ClF5 (Chlorine pentafluoride) Molecular Geometry, Bond Angles YouTube

ClF5 Lewis Structure (Chlorine Pentafluoride) YouTube

Hybridization of ClF5 (Chlorine pentafluoride) YouTube
[Solved] Draw the Lewis structure for the chlorine pentafluoride (CIF

ClF5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity

Chlorine Pentafluoride Lewis Dot Diagram First draw the lewis dot
[Solved] Draw the Lewis structure of chlorine pentafluoride. Identify
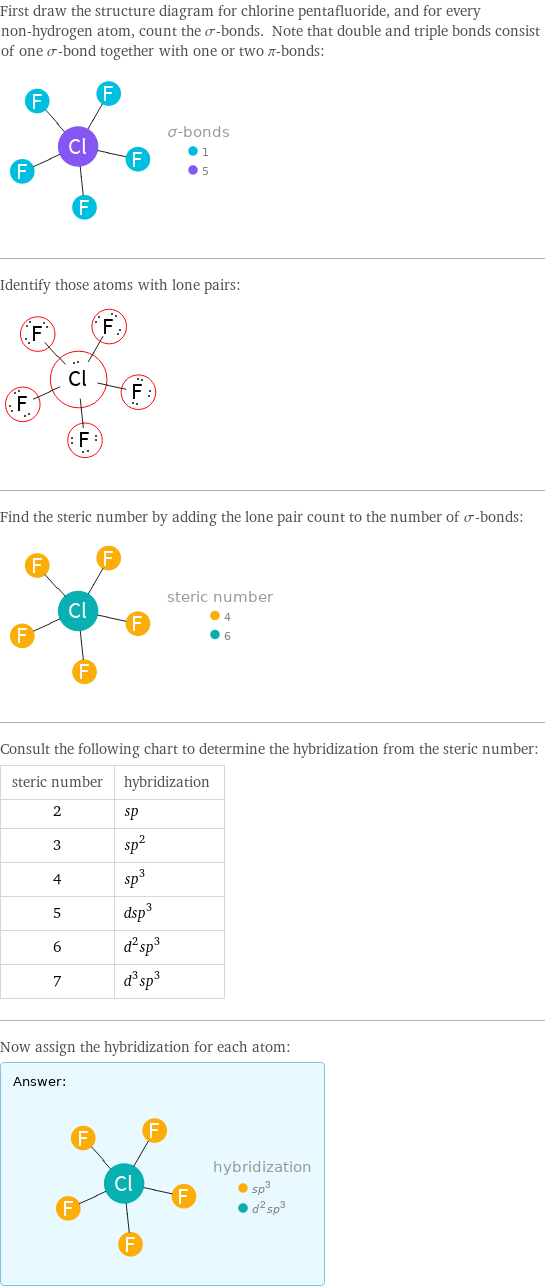
hybridization of chlorine pentafluoride
#2 Mention Lone Pairs On The Atoms.
Count The Total Number Of Valence Electrons For Chlorine And Fluorine To Use In The Lewis Structure.
This Problem Has Been Solved!
Find The Total Valence Electrons In Clf5 Molecule.
Related Post: