Draw The Lewis Structure For The Phosphorus Trichloride Molecule
Draw The Lewis Structure For The Phosphorus Trichloride Molecule - Web in pcl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Web i quickly take you through how to draw the lewis structure of pcl3, phosphorous trichloride. Draw the lewis structure for the phosphorus trichloride (pcl3) molecule. This structure is also available as a 2d mol file. Web geometry of pcl3. In this configuration, the central phosphorus atom is bonded to three chlorine atoms, and an unshared pair of electrons on phosphorus creates a pyramidal shape. A video explanation of how to draw the lewis dot structure for phosphorous trichloride,. V = 7, b = 2, n = 6. Determine the central atom in the molecule. The molecular shape of pcl3 is trigonal pyramidal. Web draw a reasonable lewis dot structure for phosphorous trichloride (pcl 3 ). In this configuration, the central phosphorus atom is bonded to three chlorine atoms, and an unshared pair of electrons on phosphorus creates a pyramidal shape. Web check me out: In order to draw the lewis structure of pcl3, first of all you have to find the total. A video explanation of how to draw the lewis dot structure for phosphorous trichloride,. This structure is also available as a 2d mol file. Determine the total number of valence electrons in the molecule by adding the valence electrons of each atom. Web pcl3 lewis structure has a phosphorus atom (p) at the center which is surrounded by three chlorine. 6.4k views 10 years ago chemistry lewis dot structures. Web draw a reasonable lewis dot structure for phosphorous trichloride (pcl 3 ). V = 7, b = 2, n = 6. Here, the given molecule is pcl3 (phosphorus trichloride). Web i quickly take you through how to draw the lewis structure of pcl3, phosphorous trichloride. This structure is also available as a 2d mol file. Web 6 steps to draw the lewis structure of pcl3 step #1: Web drawing the lewis structure for pcl3 (phosphorus trichloride) involves a series of steps to understand its molecular composition and bonding. Jmol._canvas2d (jsmol) jmolapplet0 [x] appletid:jmolapplet0_object (signed) starting hoverwatcher_1. 6.4k views 10 years ago chemistry lewis dot structures. In the complete lewis structure, the central atom of the structure is p two lone pairs of electrons around it. Web in pcl3, the formal charges are: There are 2 steps to solve this one. A) show the formation of the molecule from its constituent elements. Web to draw the lewis structure of an atom, write the symbol of the. For the pcl3 structure use the periodic table to find the. Here, the given molecule is pcl3 (phosphorus trichloride). Calculate the total number of valence electrons. V = 5, b = 6, n = 2. This structure is also available as a 2d mol file. Web 6 steps to draw the lewis structure of pcl3 step #1: Each pair of bonding electrons (:) can be represented as a single bond (|). This structure is also available as a 2d mol file. Web the pcl3 lewis structure showcases the interaction between phosphorus and chlorine atoms, revealing how they share electrons to achieve stability. 166k views 10. Calculate the total number of valence electrons. Web here are the steps to draw the pcl3 lewis structure: Web pcl3 lewis structure has a phosphorus atom (p) at the center which is surrounded by three chlorine atoms (cl). It is a volatile liquid that reacts with water and releases hcl gas. This structure is also available as a 2d mol. Web here are the steps to draw the pcl3 lewis structure: Web draw a reasonable lewis dot structure for phosphorous trichloride (pcl 3 ). Phosphorus (p) is in group 15 of the periodic table, so it has 5 valence electrons. This structure is also available as a 2d mol file. The sum of formal charges is zero, indicating a stable. Web i quickly take you through how to draw the lewis structure of pcl3, phosphorous trichloride. For the molecule phosphorus trichloride (pcl3): Draw the lewis structure for the phosphorus trichloride (pcl3) molecule. Web drawing the lewis structure for pcl3 (phosphorus trichloride) involves a series of steps to understand its molecular composition and bonding. Web draw a reasonable lewis dot structure. Web in pcl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web the pcl3 lewis structure showcases the interaction between phosphorus and chlorine atoms, revealing how they share electrons to achieve stability. Phosphorus trichloride is made up of one phosphorus atom and three chlorine atoms, having a chemical formula of pcl3. Below is the video snippet attached for the overall information of lewis structure. Determine the central atom in the molecule. Shared pairs of electrons are drawn as lines between atoms, while. Jmol._canvas2d (jsmol) jmolapplet0 [x] appletid:jmolapplet0_object (signed) starting hoverwatcher_1. Web 6 steps to draw the lewis structure of pcl3 step #1: There is 1 lone pair on the phosphorus atom (p) and 3 lone pairs on all three chlorine atoms (cl). Web in pcl3, the formal charges are: I also go over hybridization, shape and bond angle. Also, helium is shown in group 8a, but it only has two valence electrons. The molecular shape of pcl3 is trigonal pyramidal. Here’s a clear way to approach it: Web given below is the mo diagram of pf3 taking reference to which you can easily draw for pcl3.
Phosphorus trichloride lewis structure YouTube

Lewis Dot Structures Molecules Phosphorus Trichloride (PCl3) 007

Structure Phosphorous Trichloride (PCL3), Grade Standard Technical
[Solved] Draw the Lewis structure for the phosphorus trichloride P Cl 3

Phosphorus trichloride PCl₃ Molecular Geometry Hybridization

PCl3 Lewis Structure How to Draw the Lewis Structure for PCl3
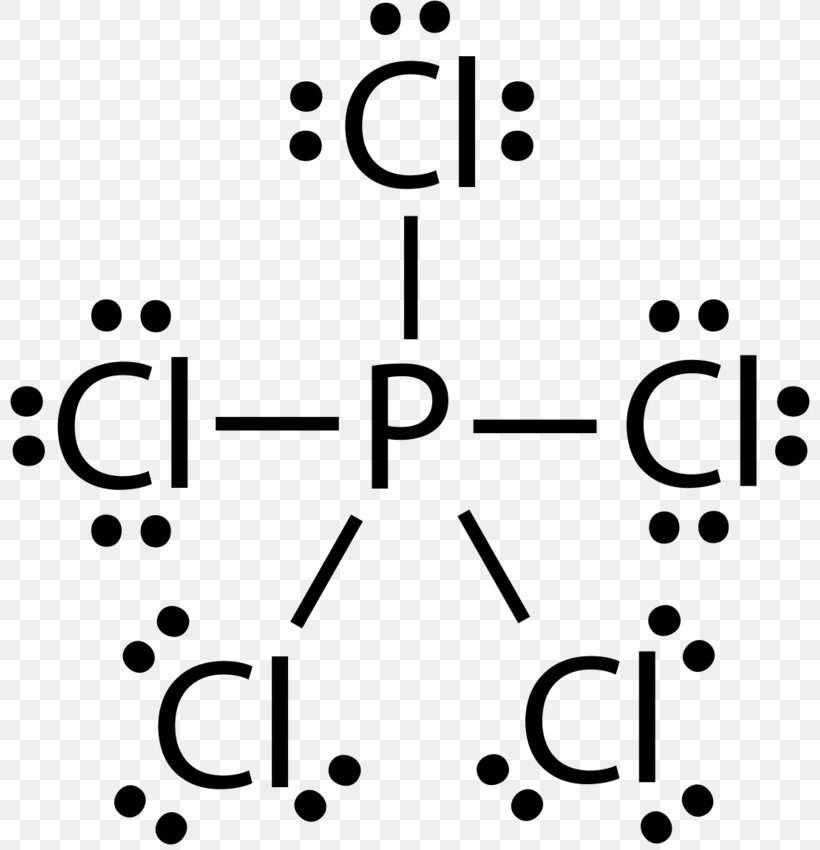
Lewis Structure Phosphorus Trichloride Phosphorus Pentachloride
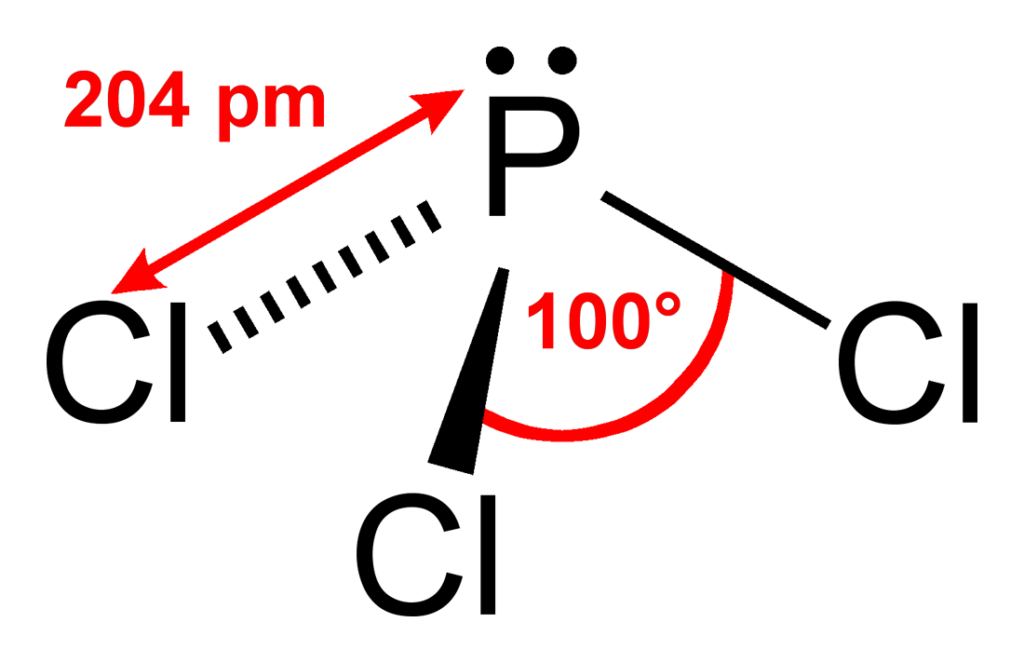
PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and

draw the lewis structure for the phosphorus trichloride(PCL3) molecule

lewis structure of phosphorus trichloride chemistryclass YouTube
Chlorine (Cl) Is In Group 17, With 7 Valence Electrons.
It Is A Volatile Liquid That Reacts With Water And Releases Hcl Gas.
Web Office Of Data And Informatics.
Also, There Is A Lone Pair On Phosphorus Atom.
Related Post: