Draw The Lewis Structure Of Ch4
Draw The Lewis Structure Of Ch4 - We're going to draw the dot structure for ch 4, carbon tetrahydride, also called methane. Web how to draw the lewis dot structure for ch4: Draw lewis structures for molecules. Assess the stability of a structure by considering formal charges of atoms. Draw resonance structures of some molecules. This widget gets the lewis structure of chemical compounds. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on. Web lewis dot structures. It has the chemical formula of ch4 and comprises one carbon atom forming bonds with four hydrogen atoms. Drawing the lewis structure for ch4. No lone pairs on valence shells of carbon and hydrogen atoms. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Assess the stability of a structure by considering formal charges of atoms. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Draw resonance structures of some molecules. For the ch4 structure use the periodic table to find the total number of. The example is for the nitrate ion. Web drawing the lewis structure for ch 4 (named methane) requires only single bonds. Here’s how you can draw its lewis structure: Post any question and get expert help quickly. How can i draw a lewis structure of a compound? Draw lewis structures for molecules. Several socratic answers give the procedure. 69k views 12 years ago every video. Draw lewis structures for molecules. 69k views 12 years ago every video. No lone pairs on valence shells of carbon and hydrogen atoms. Web carbon atom is the center atom and it is very easy to draw ch4 lewis structure. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. I also go over hybridization, shape and bond angle. There are following specifications in the lewis structure of methane. Web carbon atom is the center atom and it is very easy to draw ch4 lewis structure. Web drawing the lewis structure for. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell. Draw the lewis dot structure of a given molecule or ion. Web lewis dot structures. Web the lewis structure of ch4 contains four single bonds, with carbon in the center, and four hydrogens on. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Four hydrogen atoms have made bonds with center carbon atom and all those. Post any question and get expert help quickly. Methane is a simple molecule, consisting of one carbon atom bonded to four hydrogen atoms. 45k views 10 years ago. Web here are the steps to draw a lewis structure. Find more chemistry widgets in wolfram|alpha. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of ch4 is sp3. The carbon atom (c) is at the center and it is surrounded by 4 hydrogen atoms (h). Web here are the steps to draw a lewis structure. Answer the questions about the. Assess the stability of a structure by considering formal charges of atoms. Web lewis structure of ch4 (or methane) contains four single bonds between each carbon (c) and hydrogen (h) atoms. Not the question you’re looking for? A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. View the full answer step 2. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web drawing the lewis structure for ch 4 (named methane) requires only single bonds. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Web added jun 9, 2014 by webtester in chemistry. Here’s how to do it: Web drawing lewis structures for molecules with one central atom: Methane has four valence electrons from the carbon atom and one valence electron from each hydrogen atom, for a total of eight valence electrons. Web drawing the ch4 lewis structure is a simple and straightforward process that can be done in a few easy steps. Assess the stability of a structure by considering formal charges of atoms. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. We're going to draw the dot structure for ch 4, carbon tetrahydride, also called methane. Web lewis dot structures. Methane is one of the simple organic molecules, given its straightforward structure.
CH4 Lewis Structure, Molecular Geometry, and Hybridization

Electron Dot Diagram For Methane

In this video we are going to learn about the Lewis structure of CH4

How to Draw the Lewis Dot Structure for CH4 Methane YouTube
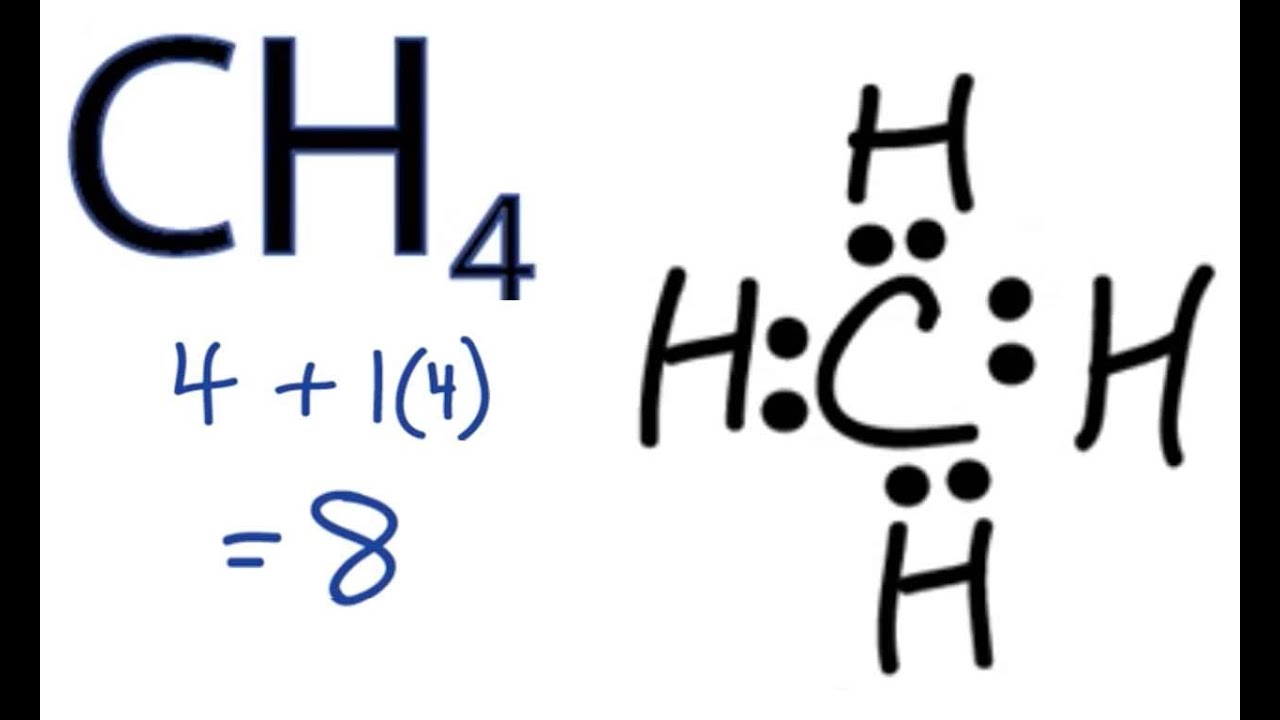
CH4 Lewis Structure How to Draw the Dot Structure for CH4 (Methane

How to draw CH4 Lewis Structure? Science Education and Tutorials

CH4 Lewis StructureLewis Structure for CH4 (Methane) YouTube

Electron Dot Diagram For Methane

Electron Dot Diagram For Methane

Draw Lewis Structure For Ch4 Nelson Tardwilis
And What We Need To Do Is Follow The Steps You See On The Screen There.
Draw Resonance Structures Of Some Molecules.
45K Views 10 Years Ago.
Web Lewis Structure Of Ch4 (Or Methane) Contains Four Single Bonds Between Each Carbon (C) And Hydrogen (H) Atoms.
Related Post: