Draw The Lewis Structure Of Hf
Draw The Lewis Structure Of Hf - Added jun 9, 2014 by webtester in chemistry. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Count the total number of valence electrons in the molecule. Hydrogen has 1 valence electron and fluorine (in group 7 with f and cl) has 7 valence electrons. Web lewis structure of hf (or hydrogen fluoride) contains one single bond between the hydrogen (h) and fluorine (f) atom. Find the total valence electrons in hf molecule. Using lewis structures to show valence electrons. For hf, we have one valence electron from hydrogen and seven valence electrons from fluorine, giving us a total of eight valence electrons. Note that each atom must contribute one electron to the bond. Remember to add one for each negative charge, and deduct one for each positive charge. #1 draw a rough skeleton structure. Web to draw the lewis structure of hf, we follow a few simple steps: Added jun 9, 2014 by webtester in chemistry. Draw out the molecular backbone of the compound. Web each atom contributes one electron to the bond. Using lewis structures to show valence electrons. For the hf structure use the periodic table to find the total number of valence electrons for the. For the hf structure use the periodic table to find the total number of valence electrons for the hf. Send feedback | visit wolfram|alpha. Web here’s how you can easily draw the hf lewis structure. Valence electrons are the electrons in the outermost shell of an atom. Web drawing the lewis structure for hf. #1 draw a rough skeleton structure. How to draw a lewis structure. We have to first count the total number of valence electrons inside a single hydrogen fluoride molecule. Using the periodic table to draw lewis dot structures. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. #3 if needed, mention formal charges on the atoms. Using lewis structures, we can represent this as follows: It has a simple structure and one can easily unders. Hydrogen belongs to period 1 and has 1 valence electron whereas fluorine. In this video, we are going to help you determine the lewis structure of the hf molecule. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Web by using the following steps, you can easily draw the lewis. Now, let’s take a closer look at each step mentioned above. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Hf has one atom of hydrogen and one atom of fluorine. It has a simple structure and one can easily unders. Hf is very similar to hf and hcl. Write lewis symbols for neutral atoms and ions. Writing lewis structures with an octet. #1 draw a rough skeleton structure. Web drawing lewis structures | introduction to solid state chemistry | materials science and engineering | mit opencourseware. In order to find the total valence electrons in hf (hydrogen fluoride) molecule, first of all you should know the valence electrons. How to draw a lewis structure. Write lewis symbols for neutral atoms and ions. Draw lewis structures depicting the bonding in simple molecules. Find more chemistry widgets in wolfram|alpha. Give examples for molecules and ions that do not follow the octet rule. Web draw the lewis dot structure of a given molecule or ion. Write lewis symbols for neutral atoms and ions. Usually, the central atom is already known as in the case with many organic compounds containing carbon as the. Using lewis structures to show valence electrons. Using the periodic table to draw lewis dot structures. Count the total number of valence electrons in the molecule. Usually, the central atom is already known as in the case with many organic compounds containing carbon as the. Send feedback | visit wolfram|alpha. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. In this structure, the hydrogen atom (h). Assess the stability of a structure by considering formal charges of atoms. Now, let’s take a closer look at each step mentioned above. Web here’s how you can easily draw the hf lewis structure step by step: Assign formal charge to an atom in a dot structure. Web lewis structure of hf (or hydrogen fluoride) contains one single bond between the hydrogen (h) and fluorine (f) atom. Note that each atom must contribute one electron to the bond. Count the total number of valence electrons in the molecule. By the end of this section, you will be able to: Two fluorine atoms can form a molecule of f 2 in the same fashion. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web drawing lewis structures | introduction to solid state chemistry | materials science and engineering | mit opencourseware. Before starting this session, you should be familiar with: This widget gets the lewis structure of chemical compounds. Find the total valence electrons in hf molecule. Hydrogen belongs to period 1 and has 1 valence electron whereas fluorine. Writing lewis structures with an octet.
So far, we’ve used 8 of the HF Lewis structure’s total 8 outermost
[Solved] Draw the lewis structure to illustrate hydrogen bonding

Hydrogen fluoride lewis structure
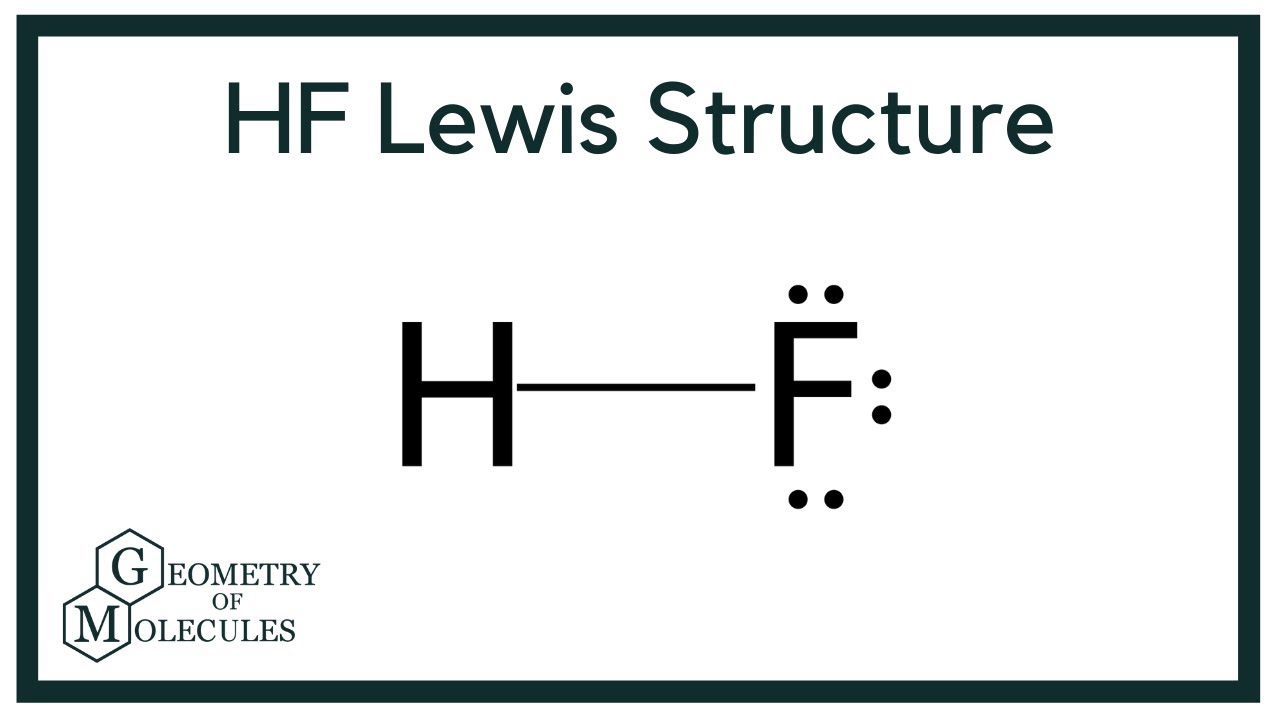
HF Lewis Structure (Hydrogen Fluoride) YouTube
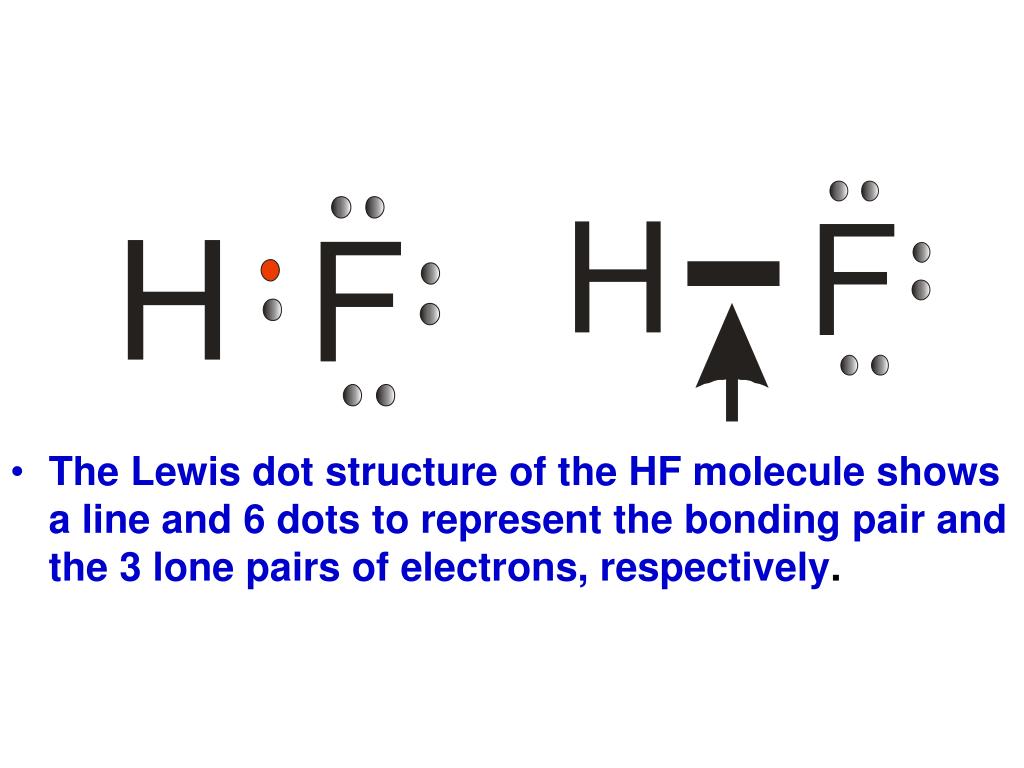
PPT Lewis Dot Structures of Covalent Compounds PowerPoint

Lewis dot structure of HF Hydrofluoric acid lewis dot structure

HF Molecular Geometry Science Education and Tutorials

HF Lewis Structure, Molecular Geometry, Hybridization, and Polarity

How to draw HF Lewis Structure? Science Education and Tutorials

Draw the Lewis Structure of HF (hydrogen fluoride) YouTube
In This Video, We Are Going To Help You Determine The Lewis Structure Of The Hf Molecule.
For Example, Two Hydrogen Atoms Can Form A Bond, Producing A Molecule Of H 2.
Draw Resonance Structures Of Some Molecules.
#2 Mention Lone Pairs On The Atoms.
Related Post: