Electrons Drawing
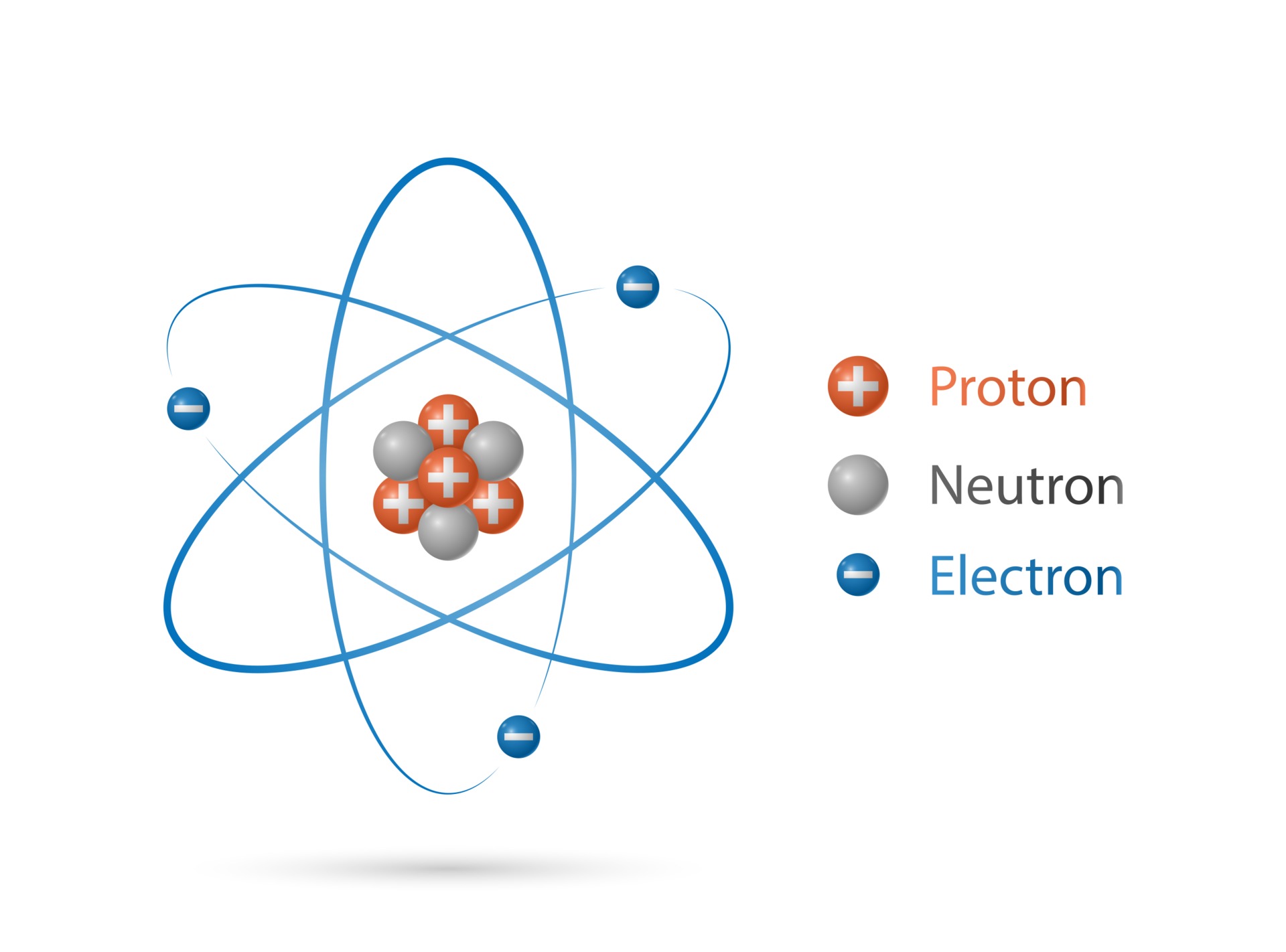
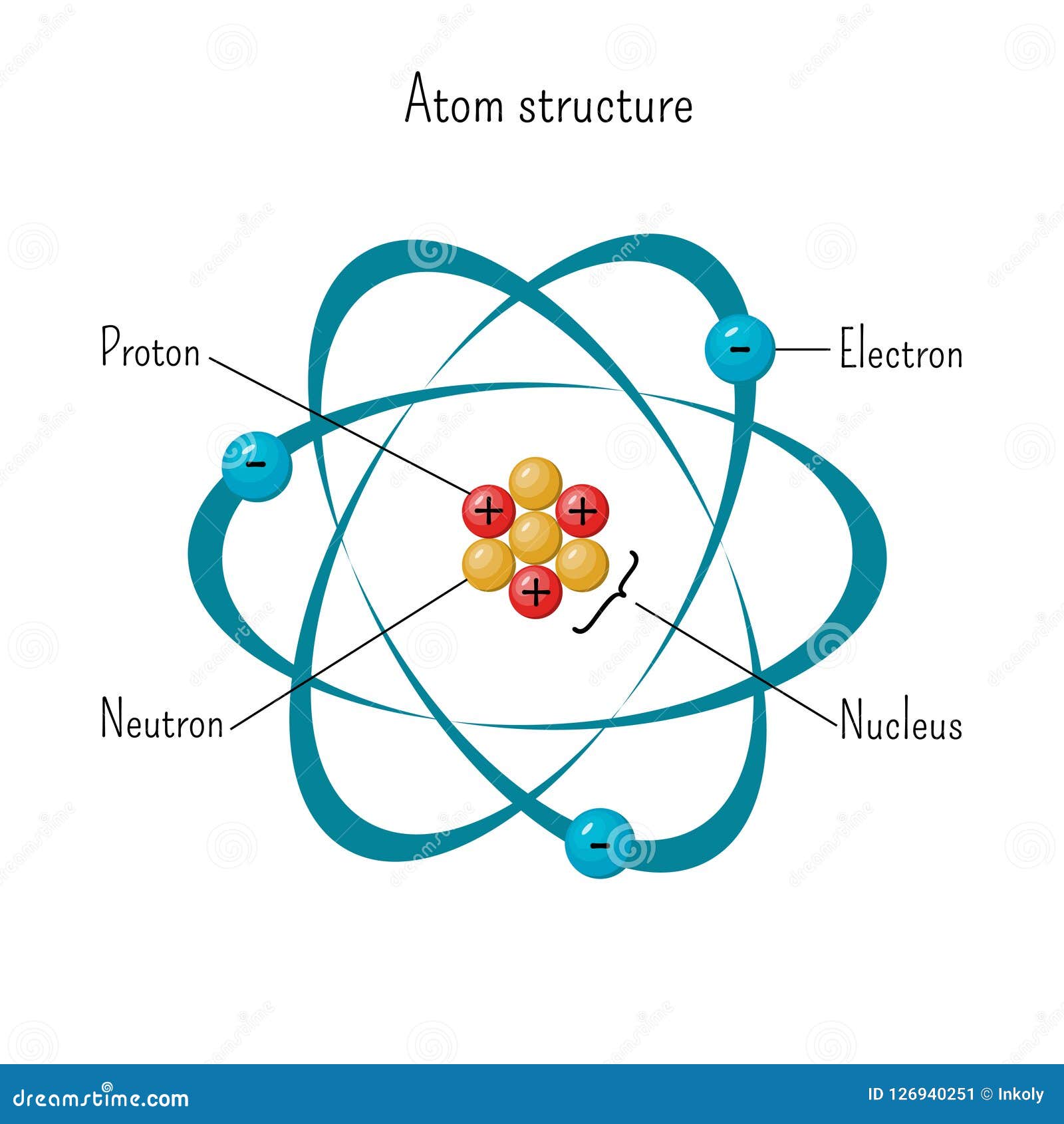
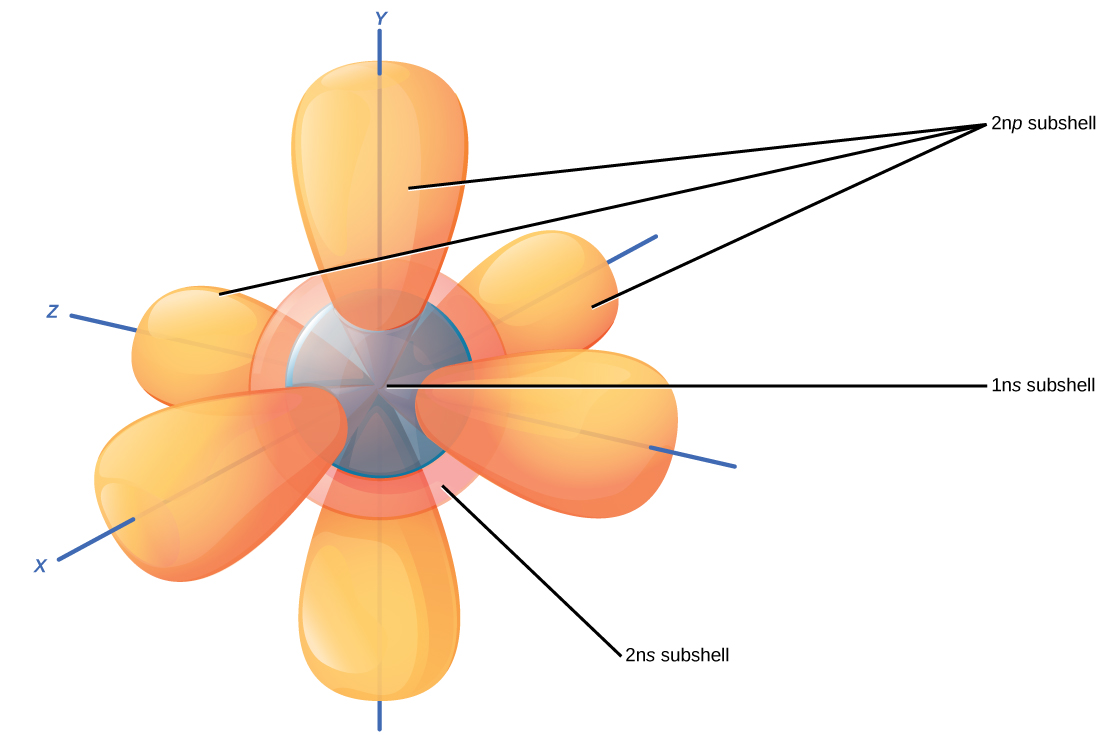
Electrons Drawing - The remaining two electrons occupy the 2p subshell. 2 electrons in the first shell. The number of dots equals. Web in this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely lewis symbols and lewis structures. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1 in. In order for an electron to be in the electron cloud of an atom, it must be in. Atoms use their electrons to participate in chemical reactions, so knowing an element’s electron configuration allows you to predict its reactivity—whether, and how, it will. Web how to draw an electron configuration diagram. Web here are the steps to draw a lewis structure. The example is for the nitrate ion. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Want to join the conversation? For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web draw lewis structures of covalent molecules. Web the best way to draw. Web when drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. Draw lewis structures for covalent compounds. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Web the best way to draw electrons is to draw them as circles with minus signs inside. Web here are the steps to draw a lewis structure. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web valence electronic structures can be visualized. Draw lewis structures for covalent compounds. Carbon (atomic number 6) has six electrons. Web specifically, an element’s position in the periodic table helps you figure out its electron configuration, how the electrons are organized around the nucleus. (hydrogen is excluded because it can hold a. When drawing the structure of an ion, be sure to add/subtract electrons to account for. Web bohr's key idea in his model of the atom is that electrons occupy definite orbitals that require the electron to have a specific amount of energy. Web draw lewis structures of covalent molecules. Web updated on november 05, 2019. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. When drawing the. The number of dots equals the number of valence electrons in the atom. An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. One of the allowable orbitals and it must have the precise energy required for that orbit. And we would account for these valence electrons in our. Web valence electronic structures can be visualized by drawing lewis symbols (for atoms and monatomic ions) and lewis structures (for molecules and polyatomic ions). Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Shell the maximum number of electrons that can fill each is: Web updated on november 05, 2019. Then play a game to. Web here's some of the guidelines for drawing dot structures. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Take sodium as an example. Web writing and drawing electronic configuration. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Web specifically, an element’s position in the periodic table helps you figure out its electron configuration, how the electrons are organized around the nucleus. And we would account for these valence electrons in our dot structure. Electron dot structures, which were discussed in the previous chapter, visually represent the valence electrons that are present in an atom as dots that. Web bohr's key idea in his model of the atom is that electrons occupy definite orbitals that require the electron to have a specific amount of energy. Web here's some of the guidelines for drawing dot structures. Web the best way to draw electrons is to draw them as circles with minus signs inside. The number of dots equals the number of valence electrons in the atom. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Web when drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling. Web specifically, an element’s position in the periodic table helps you figure out its electron configuration, how the electrons are organized around the nucleus. Web construct an atom according to the bohr model. Then play a game to test your ideas! The example is for the nitrate ion. And we would account for these valence electrons in our dot structure. It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. The remaining two electrons occupy the 2p subshell. Electrons must occupy the lowest available shell, closest to the nucleus. Electron dot structures, which were discussed in the previous chapter, visually represent the valence electrons that are present in an atom as dots that are written around an elemental symbol.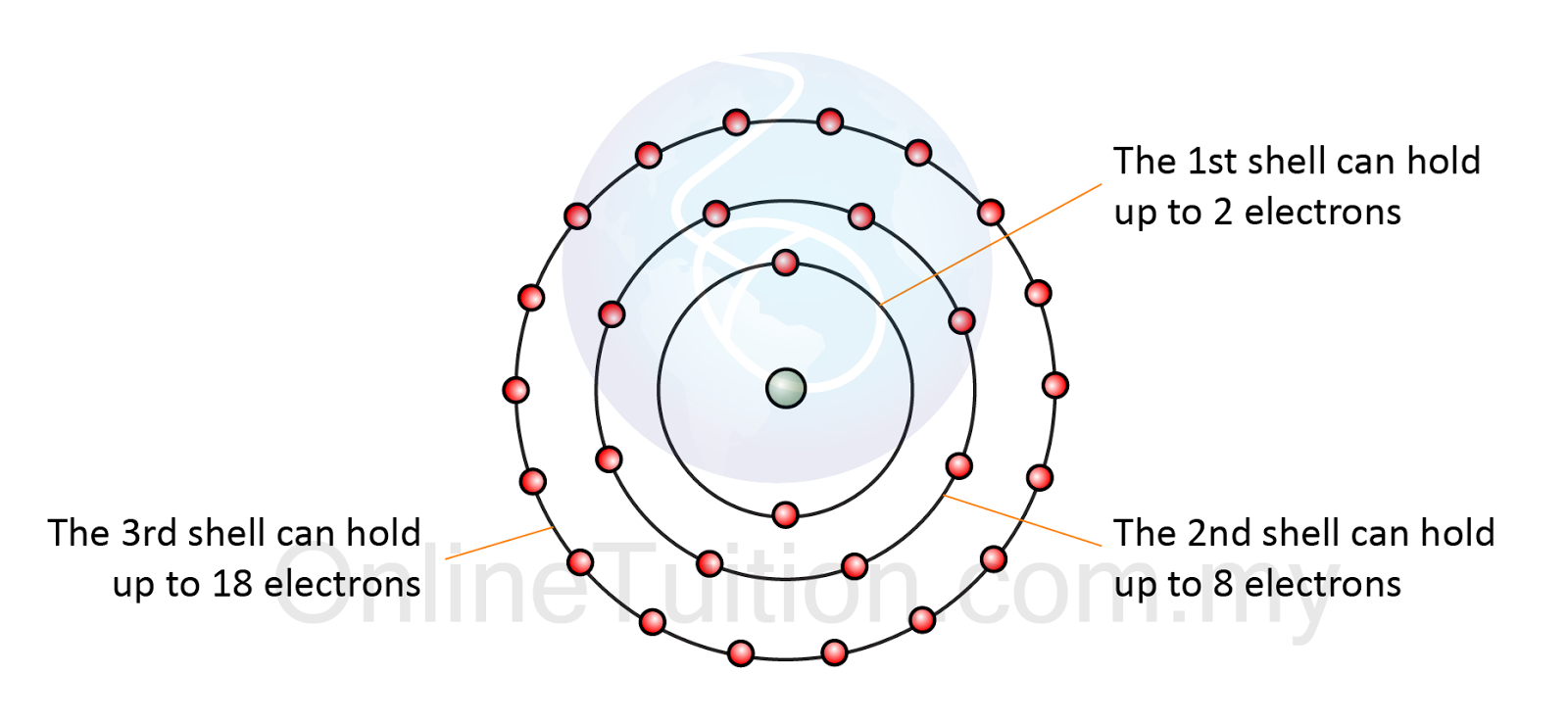
Electron Arrangement in Atom SPM Chemistry
Atomic Structure Vector Art, Icons, and Graphics for Free Download
Simple Model of Atom Structure with Electrons Orbiting Nucleus of Three
Electrons Biology for Majors I
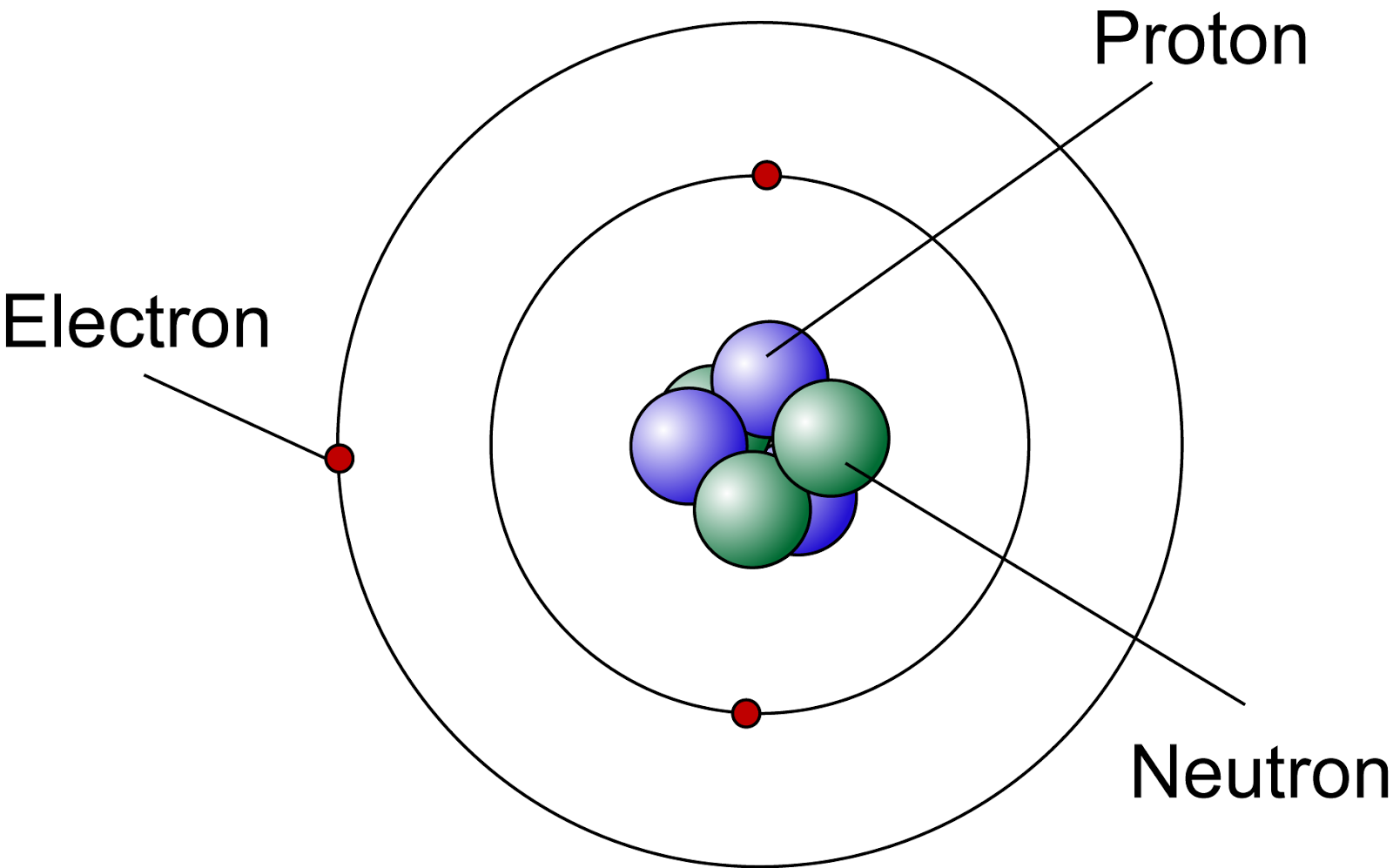
Lets Get Inside An Atom!! The Science Station
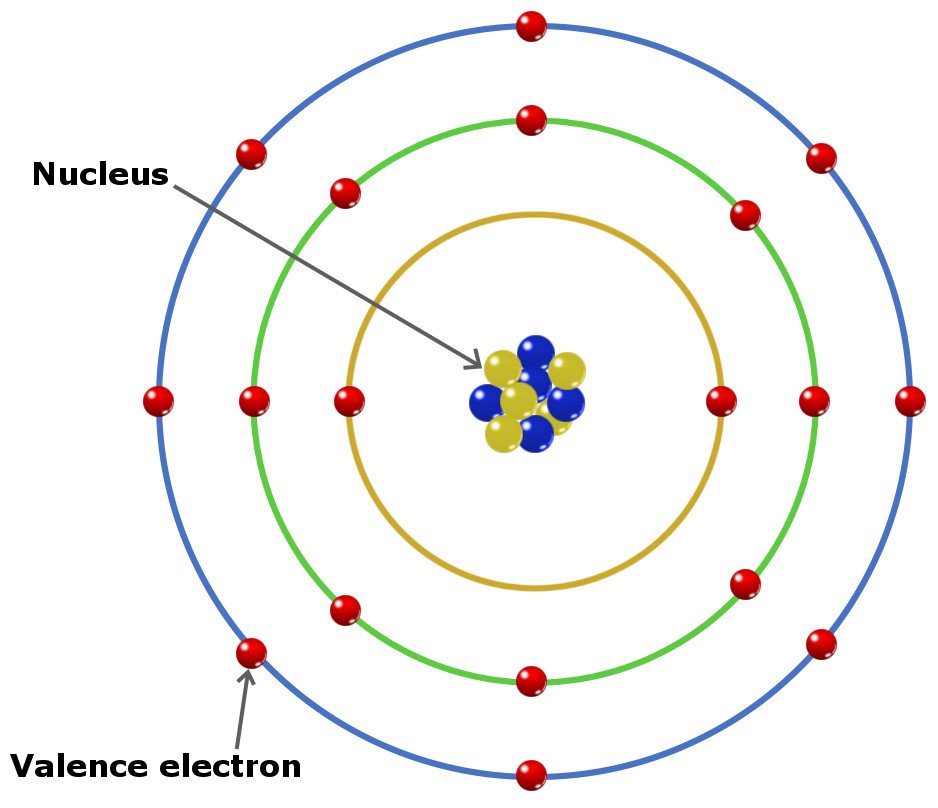
What Are Valence Electrons And How To Find Them? Where Are They Located?

Atomic Structure Broad Learnings
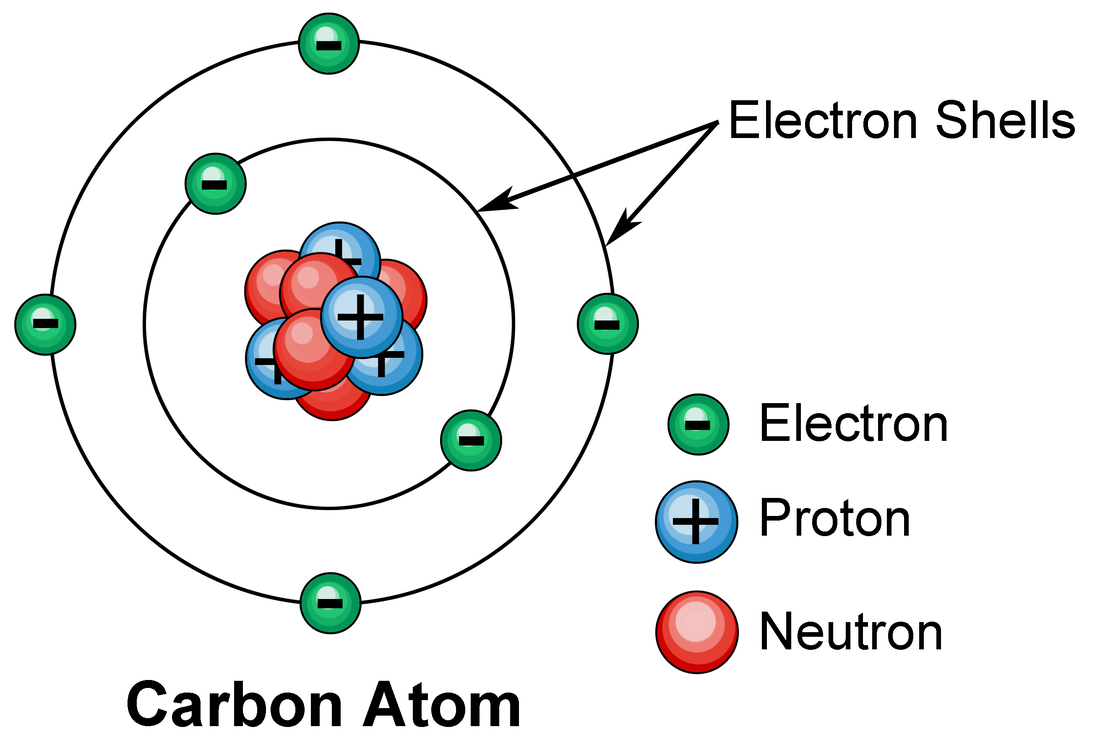
Electronic structure of matter. San Francisco de Paula, Science
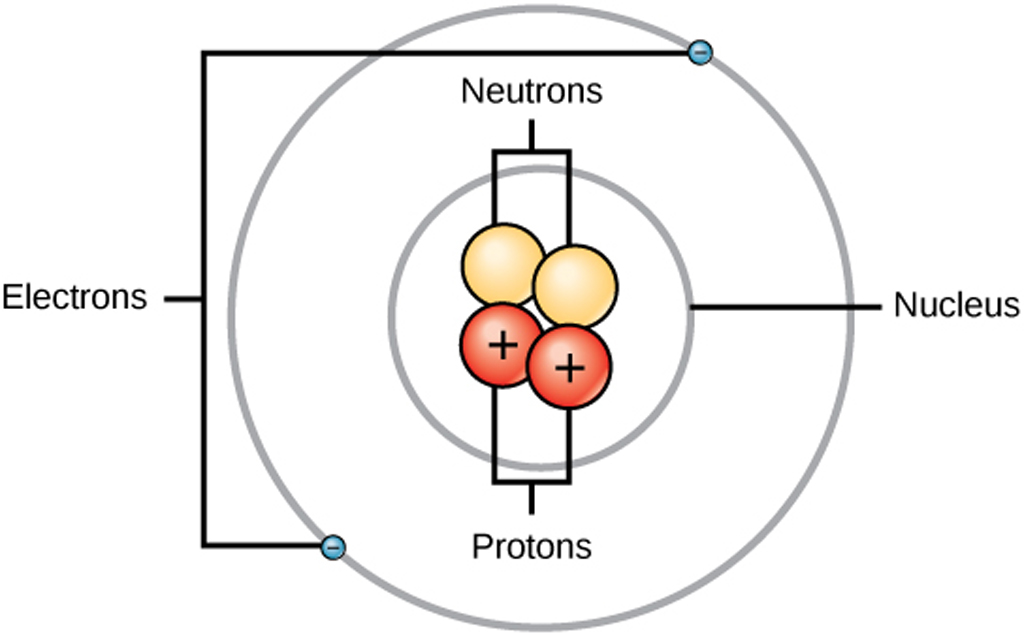
Structure of an Atom Structure & Use of Electron & Proton in Electronics
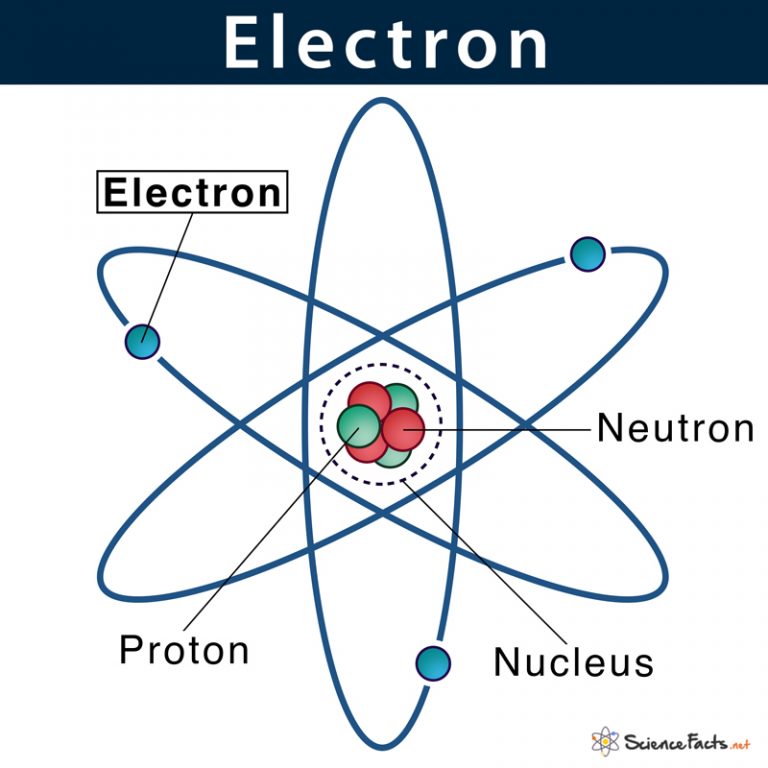
Atomic Nucleus Definition, Structure & Parts with Diagram
Web Draw Lewis Structures Of Covalent Molecules.
Web A Lewis Electron Dot Symbol (Or Electron Dot Diagram Or A Lewis Diagram Or A Lewis Structure) Is A Representation Of The Valence Electrons Of An Atom That Uses Dots Around The Symbol Of The Element.
Web Writing And Drawing Electronic Configuration.
One Of The Allowable Orbitals And It Must Have The Precise Energy Required For That Orbit.
Related Post: