How Do You Draw A Water Molecule
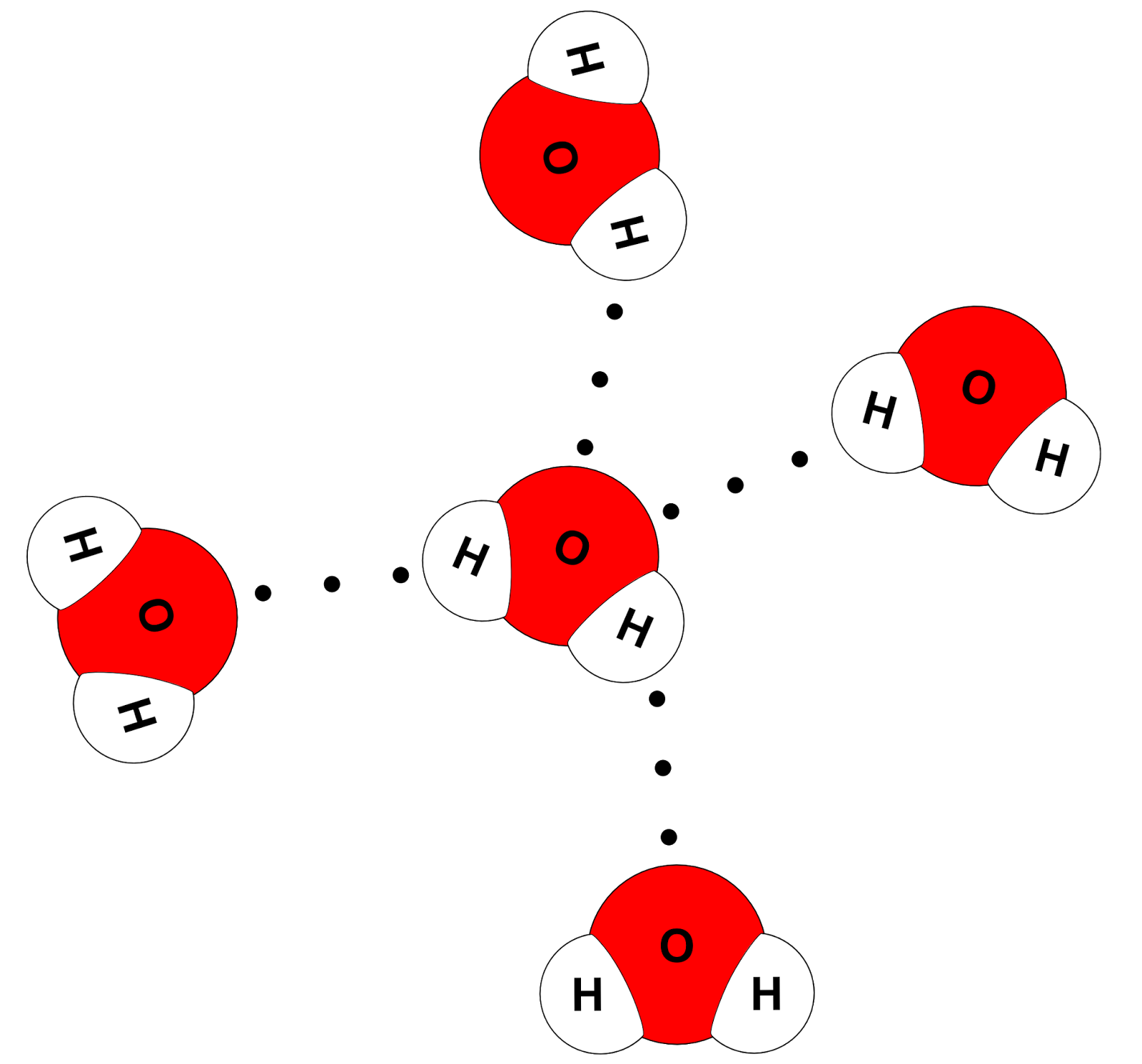
How Do You Draw A Water Molecule - Chemists normally represent a bond using a line instead of two dots. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Each step of drawing lewis structure of h 2 o are explained in this tutorial. Μ = q × d. 316k views 12 years ago. Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. How do they interact with each other through hydrogen bonding? Ever seen a water molecule? This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. The atomic structure of water. One color to represent the oxygen atom and one color to represent the two hydrogen atoms. Learn more about how the structure of a water molecule makes it so versatile. In a water molecule, the oxygen atom and hydrogen atoms share electrons in covalent bonds, but the sharing is not equal. Web each water molecule links to four others creating. Web hydrogen bonding in water. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). The atomic structure of water. Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. Oxygen is highly electronegative, which creates a partial negative. Web a molecule of water has one oxygen atom covalently bonded to 2 hydrogen atoms. In the covalent bond between oxygen and hydrogen, the oxygen atom attracts electrons a bit more strongly than the. Where q is the amount of charge and d is the distance between the two charges. The magnitude of the turning force is given by the. Oxygen is highly electronegative, which creates a partial negative charge on one end of the molecule, and a partial positive charge on the other. How do they interact with each other through hydrogen bonding? Ever seen a water molecule? Web canadian museum of nature. Students will also be able to show in a drawing that the polar nature of water. For the h2o structure use the periodic table to find the total number of valence electrons for the h2o molecule. Web the water molecule, visualized three different ways: Web the bent shape of water molecules gives them both negative and positive sides. Chemists normally represent a bond using a line instead of two dots. 316k views 12 years ago. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Chemists normally represent a bond using a line instead of two dots. Web a quick explanation of the molecular geometry of h2o (water) including a description of the h2o bond angles.looking at the h2o lewis structure we can see tha. Because of the. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms. Μ = q × d. Students will also be able to show in a drawing that the polar nature of water can explain. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid. Water is an excellent solvent. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Push the. How do they interact with each other through hydrogen bonding? For the h2o structure use the periodic table to find the total number of valence electrons for the h2o molecule. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. One color to represent the oxygen atom and one color. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Covalent bonds being. 316k views 12 years ago. The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid. Covalent bonds being chemical bonds that are formed by the sharing of one or more pairs of electrons by the outer. The magnitude of the turning force is given by the formula. Web #tutorials #chemistry #lessonsin this video, we will draw dot and cross diagram of water molecule. From there, we will determine the structural formula of wa. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. The structures of h 2 , f 2 , and h 2 o would usually be drawn as follows: Web the water molecule, visualized three different ways: Web canadian museum of nature. Web the bent shape of water molecules gives them both negative and positive sides. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about.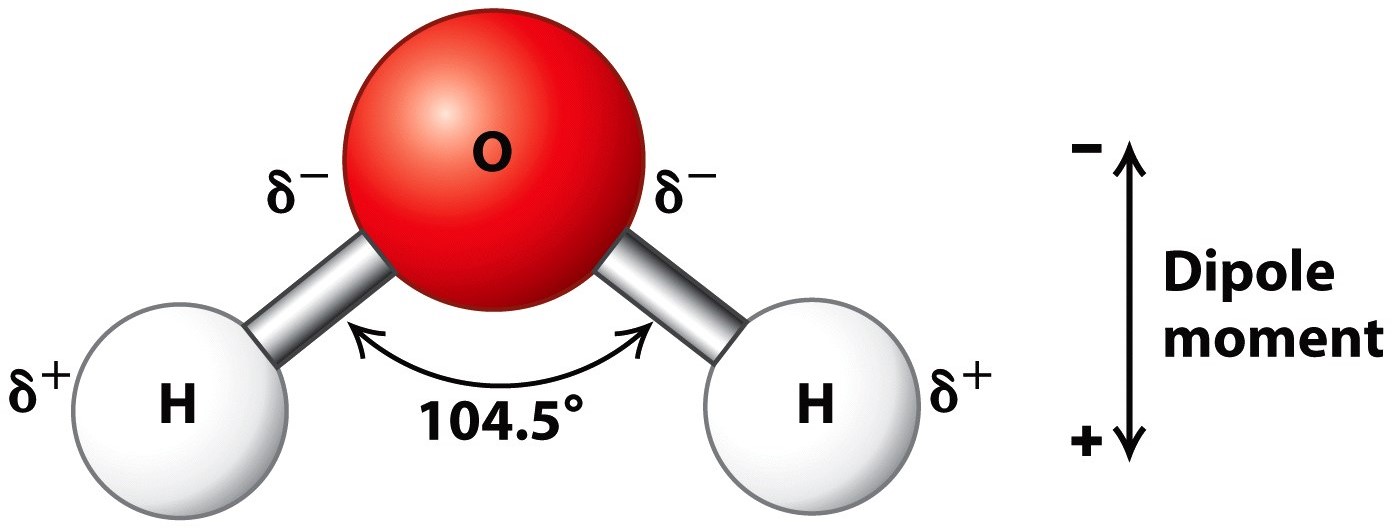
Describe the Structure of a Water Molecule
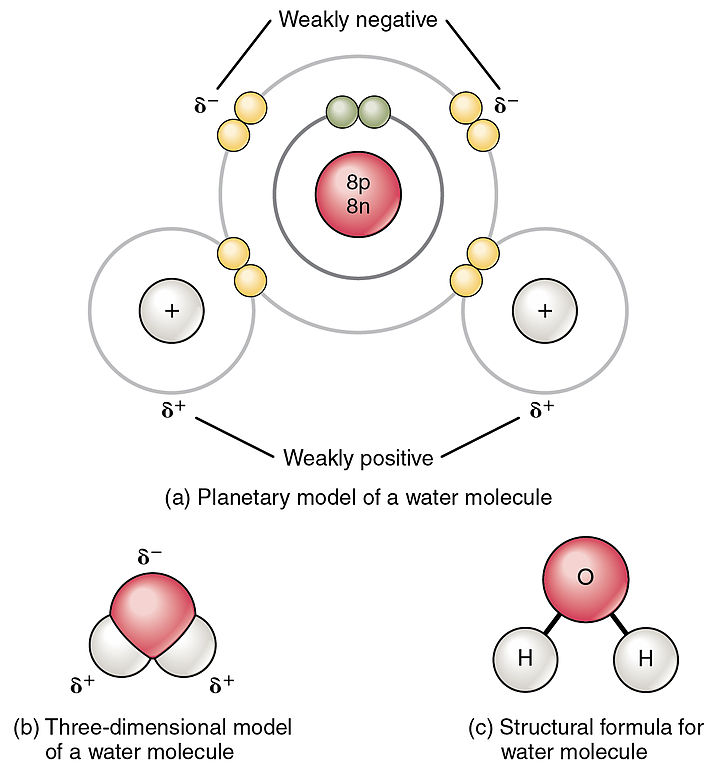
Water Principles of Biology
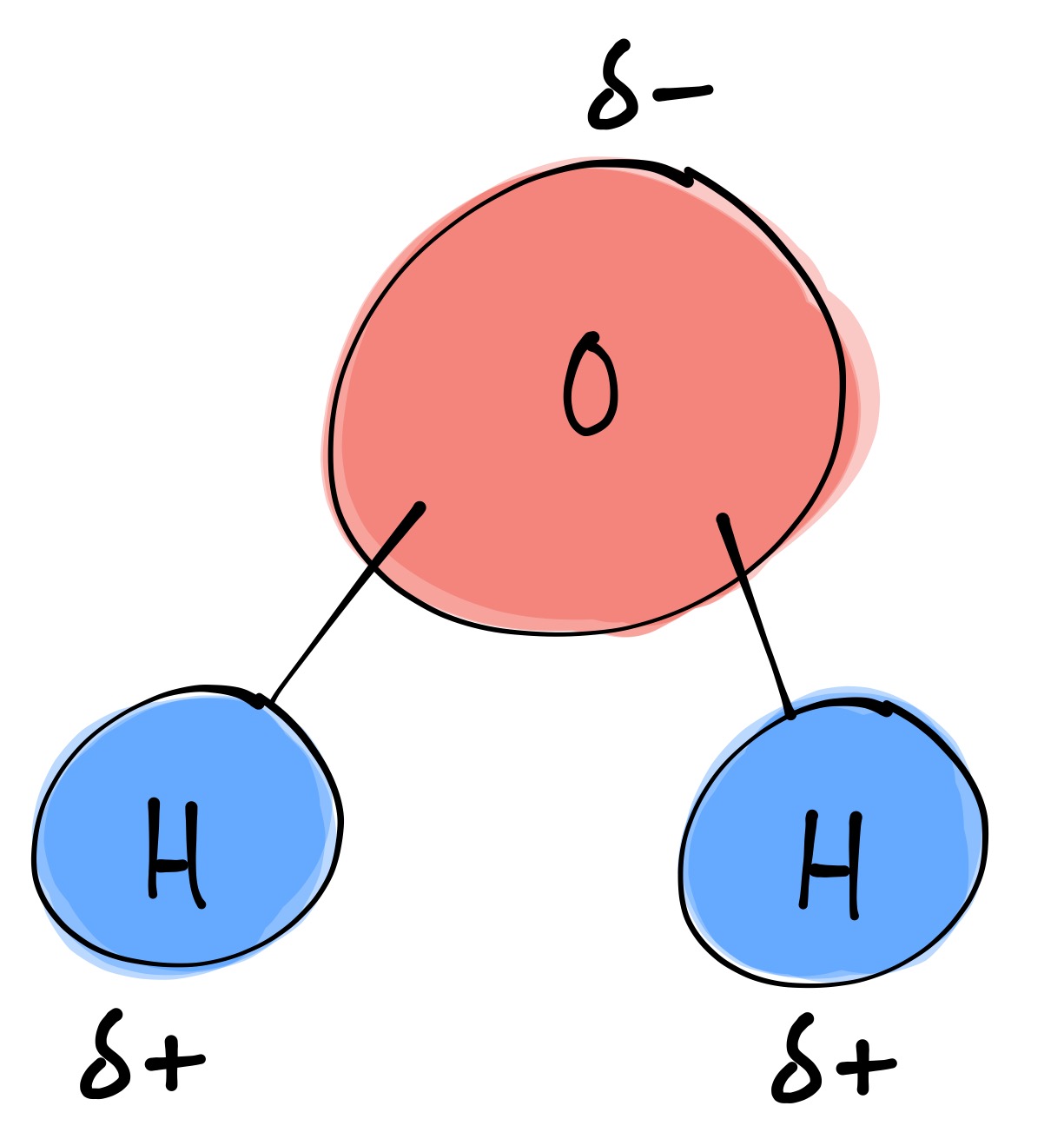
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule

H2O Lewis Structure, Molecular Geometry, and Hybridization

Types of Atoms Science at Your Doorstep
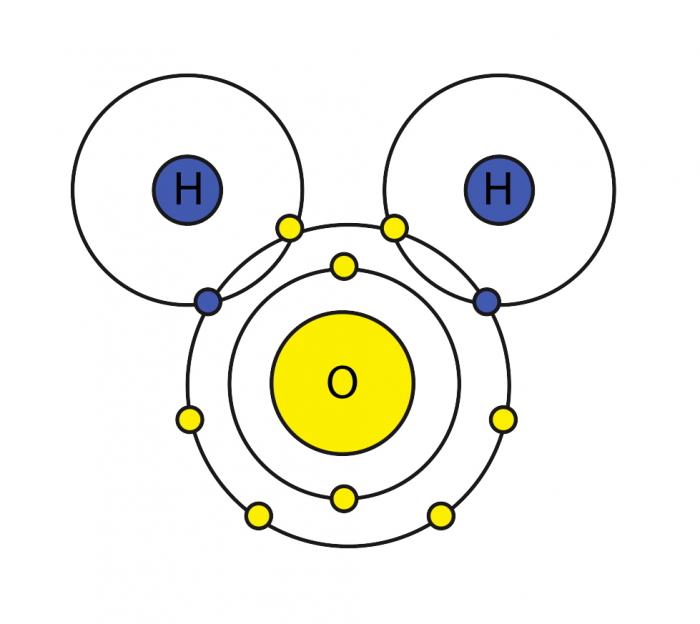
The Configuration of the Water Molecule EARTH 111 Water Science and
Science online The importance of the water and its structure
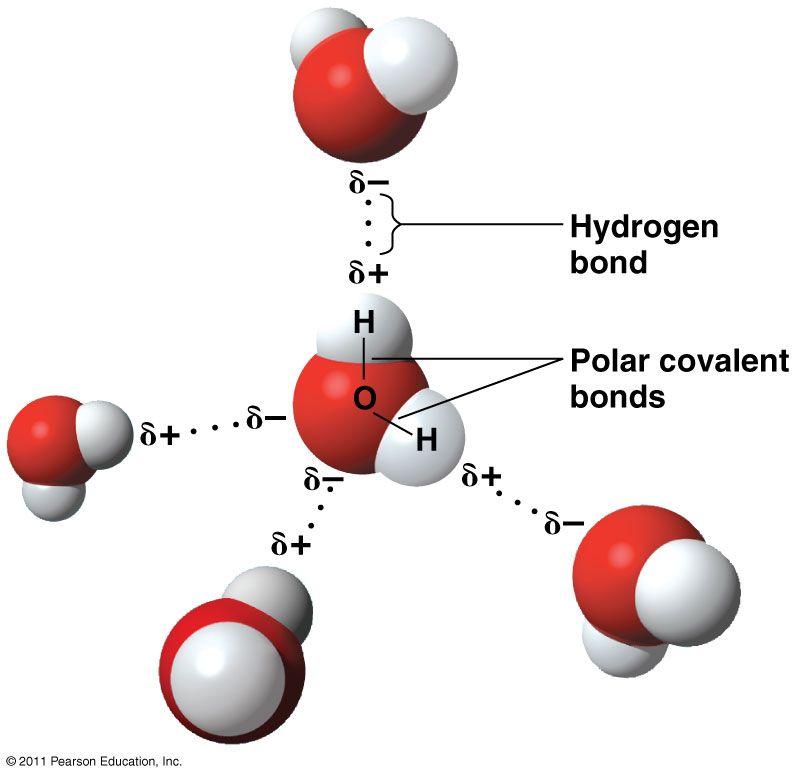
Diagram Of Water Molecule Labeled
Chemistry model of molecule water H2O scientific elements. Integrated
Diagram Of Water Molecule Labeled vrogue.co
Electronic Structure And Covalent Bonding.
Number Of Total Valence Electrons Of Oxygen And Hydrogen Atoms Are Used To Draw Lewis Structure.
Identify Three Special Properties Of Water That Make It Unusual For A Molecule Of Its Size, And Explain How These Result From Hydrogen Bonding.
Μ Is The Turning Moment.
Related Post: