How Do You Draw An Ion
How Do You Draw An Ion - Lewis structure for ionic compounds. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Web to find the electron configuration for an ion, first identify the configuration for the neutral atom. Remember that lewis dot structures. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Most elements form only one or the other. Web a molecular orbital diagram for a diatomic molecule (two atoms) varies in the number of electrons. If you are using this for ncea or revising for exams, you may be more interested in the exam guide which focuses. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1 in the previous section. This chemistry video explains how to draw the lewis structures of ionic compounds. Web this tutorial shows you how to convert diagrams of atoms into diagrams of ions. If you are using this for ncea or revising for exams, you may be more interested in the exam guide which focuses. How do you populate the electrons? As mentioned above, there are positive and negative ions. Web a molecular orbital diagram for a diatomic. 3.1 lewis structure of a cation. How to draw lewis dot structure? Web common charges of ions formed by elements in different groups of the periodic table. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. The valence electron configuration of thallium, whose symbol is tl, is 6 s2 5 d10 6 p1. 3.1 lewis structure of a cation. Drawing lewis dot structures for polyatomic ions. What is the lewis electron dot diagram for the tl + ion? Examples for drawing lewis structures for covalent bonds. 1.1 what does a lewis dot structure represent? Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1 in the previous section. Web draw lewis structures depicting the bonding in simple molecules. The valence electron configuration of thallium, whose symbol is tl, is 6 s2 5 d10 6 p1. 2.3k views 3 years ago. What is a. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Remember that lewis dot structures. Web a molecular orbital diagram for a diatomic molecule (two atoms) varies in the number of electrons. A (in anion) becomes before c (cation) in the alphabet. 2.3k views 3 years ago. What is the lewis electron dot diagram for the tl + ion? Web during ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Lewis structure for ionic compounds. Web write a negative sign as a superscript, followed by the number of electrons the atom has gained or write a positive sign as. If you are using this for ncea or revising for exams, you may be more interested in the exam guide which focuses. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. What is the lewis electron dot diagram for the tl + ion? Web draw lewis structures for ionic compounds.. The valence electron configuration of thallium, whose symbol is tl, is 6 s2 5 d10 6 p1. How to draw a lewis structure. Figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. A (in anion) becomes before c (cation) in the alphabet. 292k views 3 years ago new ap & general chemistry video playlist. Then, add or remove electrons depending on the ion's charge. Remember that lewis dot structures. Web common charges of ions formed by elements in different groups of the periodic table. Hydrogen is somewhat unusual in that it readily forms both cations and anions. A sodium atom has 1 electron in its outer shell. 3.1 lewis structure of a cation. What is the lewis electron dot diagram for the tl + ion? Web draw lewis structures depicting the bonding in simple molecules. Magnesium now has an empty third shell so draw the second shell instead. Web draw lewis structures for ionic compounds. Practice with drawing lewis structures. Web when drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. The calcium ion would be. Web bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Web a molecular orbital diagram for a diatomic molecule (two atoms) varies in the number of electrons. Web using lewis structures to show valence electrons. Most elements form only one or the other. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Then, add or remove electrons depending on the ion's charge. Hydrogen is somewhat unusual in that it readily forms both cations and anions. This video explains how to draw the electron configuration of an ion of sodium, as well as many other representations of. The valence electron configuration of thallium, whose symbol is tl, is 6 s2 5 d10 6 p1. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1 in the previous section. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the structure of ionic compounds, 2.3k views 3 years ago.
Formation of Ion SPM Chemistry
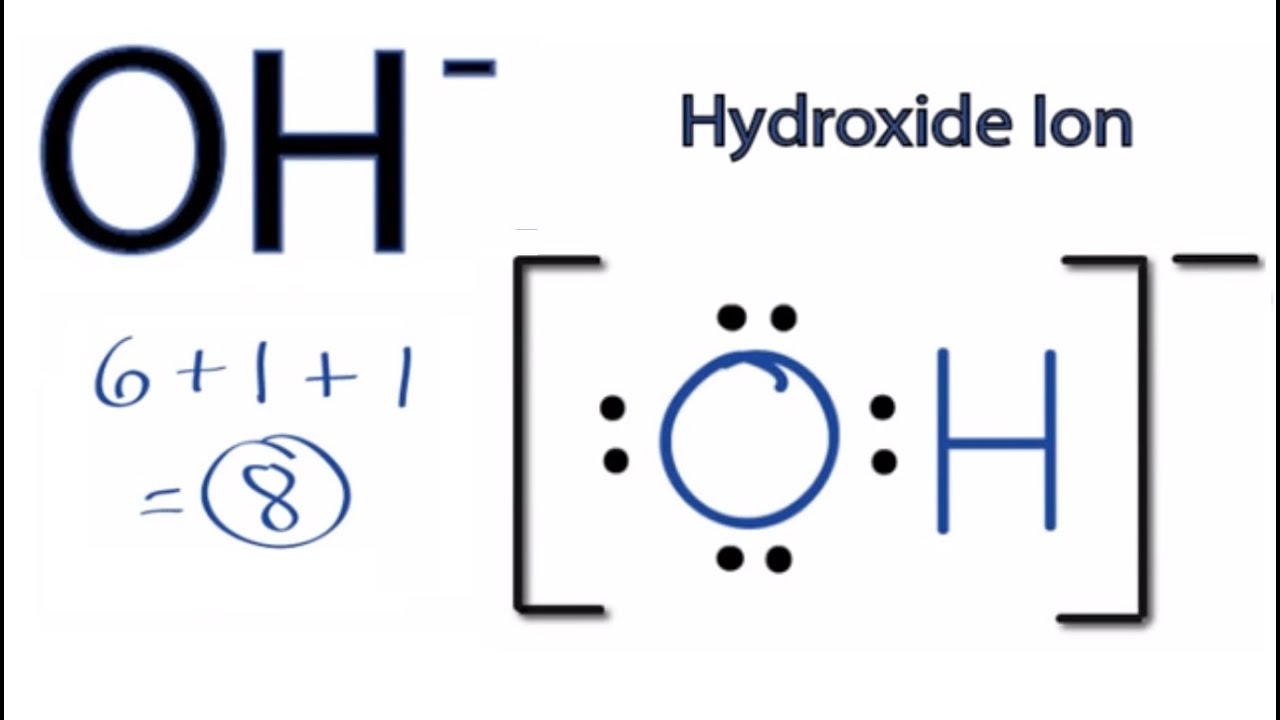
OH Lewis Structure How to Draw the Lewis Dot Structure for the
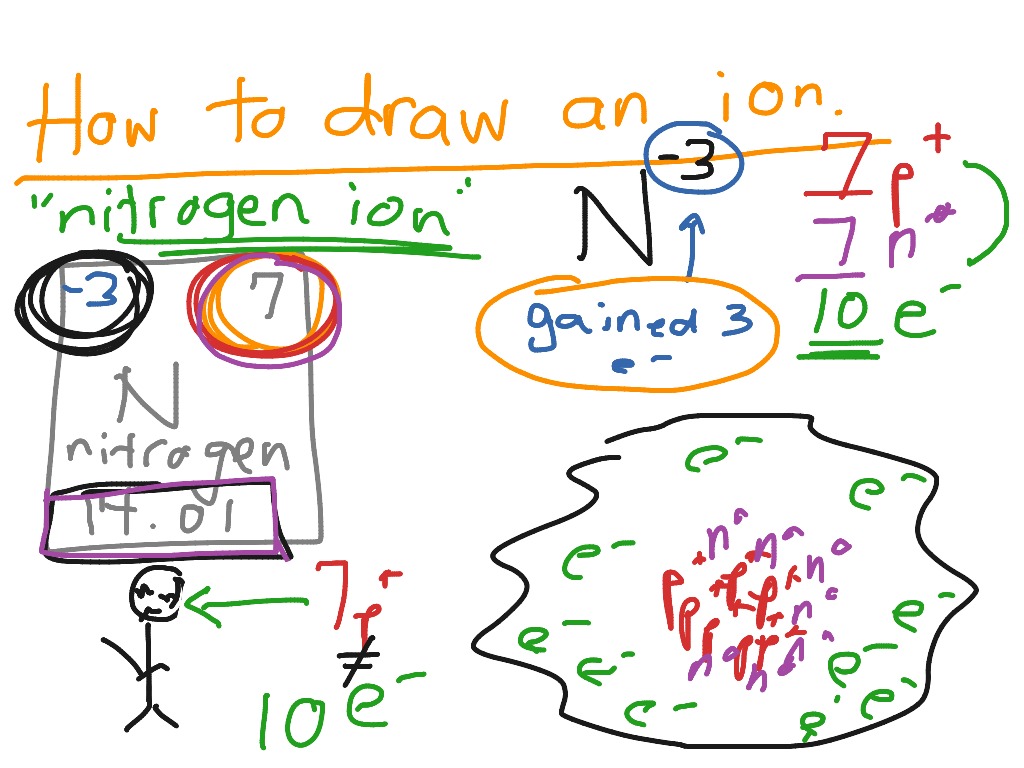
ShowMe how to draw an ion

What are Ionic Compounds and how they are formed?

How to Draw an Ion YouTube

Drawing Ions (NCEA L1 & Junior Science) YouTube
Ion atom molecule education poster Royalty Free Vector Image
Ion Definition and Examples Biology Online Dictionary
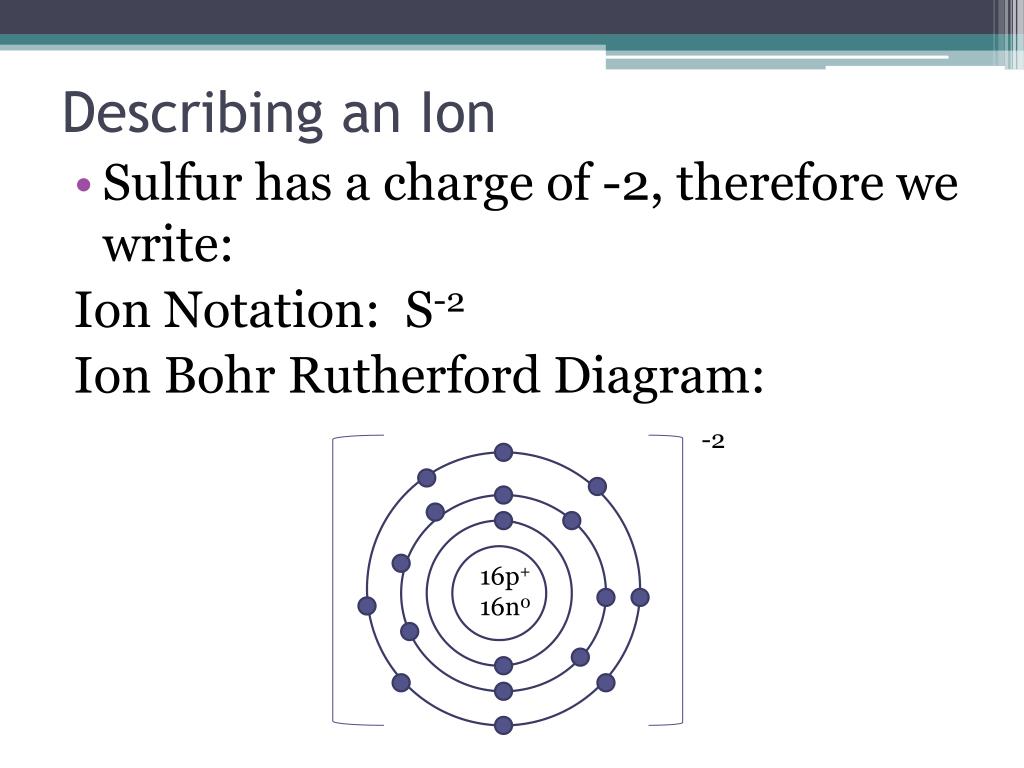
How To Draw A Bohr Model For An Ion

Lewis Dot Structure Ionic Compounds
If You Are Using This For Ncea Or Revising For Exams, You May Be More Interested In The Exam Guide Which Focuses.
What Happens When A Sodium Atom Becomes A Sodium Ion?
Many Of The Ions That Form Have Eight Electrons In Their Valence Shell.
The Astute Reader May Have Noticed Something:
Related Post: