How To Draw Lewis Dot Diagrams
How To Draw Lewis Dot Diagrams - Web how to draw lewis structures. How to draw electron dot structures? What are some examples of lewis structures? Draw the valence electrons around each atom. Finally, you'll understand all those weird pictures of molecules with the. 673k views 5 years ago lewis. Add an electron for each negative charge. What are lewis dot structures used for? What is an example of a lewis structures practice problem? Web lewis structures made easy: What are some examples of lewis structures? Assign formal charge to an atom in a dot structure. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Draw a lewis electron dot diagram for an atom or a monatomic ion. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or. 1m views 13 years ago chemistry. These symbols will represent the atoms present in the covalent bond. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Thus far, we have discussed the various types of bonds that form between atoms and/or ions. Also, helium is shown in group 8a, but it only has. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. The video covers the basic lewis structures you'll see in an introductory. Web here are the steps to draw a lewis structure. The octet rule is introduced and. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Finally, you'll understand all those weird pictures of molecules with the. Assess the stability of a structure by considering formal charges of atoms. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Enter the formula of the. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web lewis structures made easy: 2.8m views 10 years ago lewis structures. The octet rule is introduced and explain. It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Also, helium is shown in group 8a, but it only has two valence electrons. Valence electrons are represented as dots. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. If the. The video covers the basic lewis structures you'll see in an introductory. Add an electron for each negative charge. 673k views 5 years ago lewis. Write the atomic symbol for each atom. And we would account for these valence electrons in our dot structure. If the compound or molecule is charged e.g. Assign formal charge to an atom in a dot structure. How to draw electron dot structures? What is the lewis structure for so2? When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Web here are the steps to draw a lewis dot diagram: Chad explains and demonstrates exactly how to draw lewis dot structures in this lesson including numerous examples. Examples for drawing lewis structures for covalent bonds. How to draw a lewis structure. The example is for the nitrate ion. Web creating lewis diagrams is rather simple and requires only a few steps and some accounting of the valence electrons on each atom. The first thing we would need to do is to find the total number of valence electrons. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.. Draw a lewis electron dot diagram for an atom or a monatomic ion. Draw resonance structures of some molecules. If the compound or molecule is charged e.g. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. How do you draw the lewis structure for ions? Using lewis structures to show valence electrons. The video covers the basic lewis structures you'll see in an introductory. Assign formal charge to an atom in a dot structure. The number of dots equals. The first thing we would need to do is to find the total number of valence electrons. 698k views 13 years ago. The example is for the nitrate ion. Then we write the rest of the formula being. Professor dave here, let's learn how to draw lewis dot structures. Examples for drawing lewis structures for covalent bonds. What are lewis dot structures used for?
How to draw Lewis Structures a step by step tutorial Middle School

How to Draw a Lewis Structure

3 Ways to Draw Lewis Dot Structures wikiHow

How To Draw Lewis Dot Diagrams Simplereality27

How To Draw Lewis Structures A Step By Step Tutorial
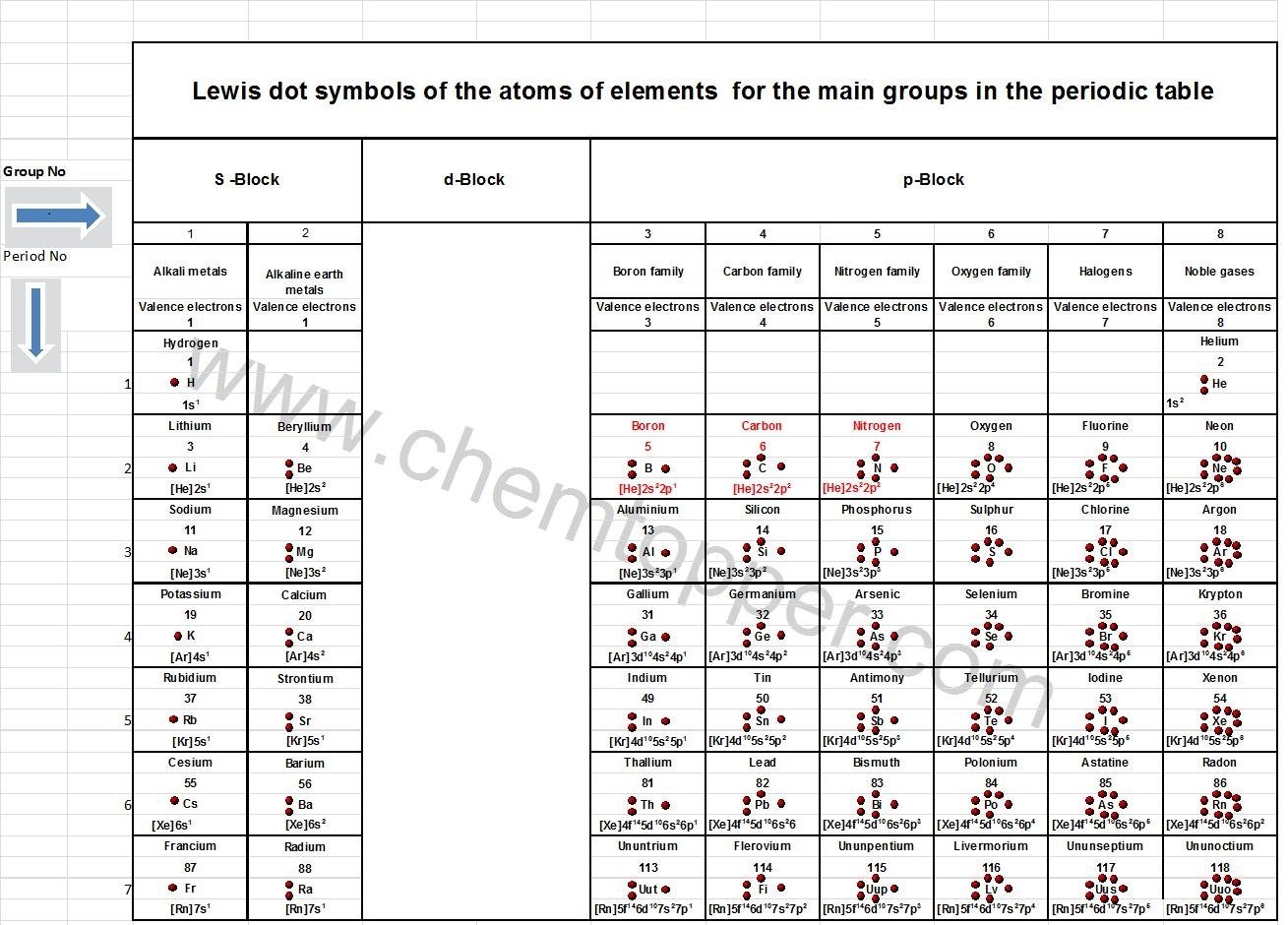
How to Draw Lewis Dot Structure Online Chemistry Tutor

Lewis Diagrams Made Easy How to Draw Lewis Dot Structures Watch

Lewis Dot Structure Definition, Examples, and Drawing

Molecular Modeling Digital and Analog General Chemistry Lab News

3 Ways to Draw Lewis Dot Structures wikiHow
Assess The Stability Of A Structure By Considering Formal Charges Of Atoms.
A Video Tutorial For How To Draw Lewis Structures In Five Steps.
4.5M Views 7 Years Ago Lewis Structure Tutorial.
Web Here's Some Of The Guidelines For Drawing Dot Structures.
Related Post: