How To Draw The Lewis Dot Diagram
How To Draw The Lewis Dot Diagram - The number of dots equals. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. Web how to draw lewis structures. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Assign formal charge to an atom in a dot structure. Give examples for molecules and ions that do not follow the octet rule. This chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as cl2,. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be nacl, whose lewis structure is: 698k views 13 years ago. Web the lewis dot diagram for the covalent bonding of chlorine, ( cl2 ), would be: Ask me questions on facebook: Web these diagrams are helpful because they allow us to show how atoms are connected, and when coupled with valence shell electron repulsion theory (vsepr), we can use lewis structures to predict the shape of the molecule. A lewis. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. Stages to articulate the electron dot formula are stated beneath. Draw a lewis electron dot diagram for an atom or a monatomic ion. This chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as cl2,. Web. Web a lewis structure is a graphic representation of the electron distribution around atoms. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. What is an example of a lewis structures practice problem? The example is for the nitrate ion. Using lewis structures to show valence electrons; Web using the periodic table to draw lewis dot structures; How do you draw the lewis structure for ionic compounds? A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Using lewis structures to show valence electrons; Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. Draw a lewis electron dot diagram for an atom or a monatomic ion. Stages to articulate the electron dot formula are stated beneath. Lewis dot structure of atoms comprising ammonia and the dot structure of the. Examples for drawing lewis structures for covalent bonds ; Andersen shows you how to draw lewis dot diagrams for atoms. Web using the periodic table to draw lewis dot structures; Be sure to leave enough space between the atoms to draw your electrons and bonds. How do you draw the lewis structure for ionic compounds? These symbols will represent the atoms present in the covalent bond. Ask me questions on facebook:. When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Assess the stability of a structure by considering formal charges of atoms. Give examples for molecules and ions that do not follow the octet rule. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Web a lewis structure is a graphic representation of the electron distribution around atoms. Give examples for molecules and ions that do not follow the octet rule. Web a video tutorial for how to draw lewis structures in five steps.. Practice with drawing lewis structures To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Web a. Web the lewis dot diagram for the covalent bonding of chlorine, ( cl2 ), would be: To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. How do you draw the lewis structure. A lewis structure also helps to make a prediction about the geometry of a molecule. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Lewis dot structure of atoms comprising ammonia and the dot structure of the molecule. Using lewis structures to show valence electrons; The example is for the nitrate ion. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web how to draw lewis structures. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. The number of dots equals. Web a video tutorial for how to draw lewis structures in five steps. Web the lewis dot diagram for the covalent bonding of chlorine, ( cl2 ), would be: Assign formal charge to an atom in a dot structure. How to draw a lewis structure; The video covers the basic lewis structures you'll see in an introductory chemistry class. Practice with drawing lewis structures
3 Ways to Draw Lewis Dot Structures wikiHow

3 Ways to Draw Lewis Dot Structures wikiHow

How to Draw a Lewis Structure

How to Draw a Lewis Structure
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How to Draw a Lewis Structure

BohrRutherford Diagrams & Lewis Dot Diagrams Eve Wongworakul
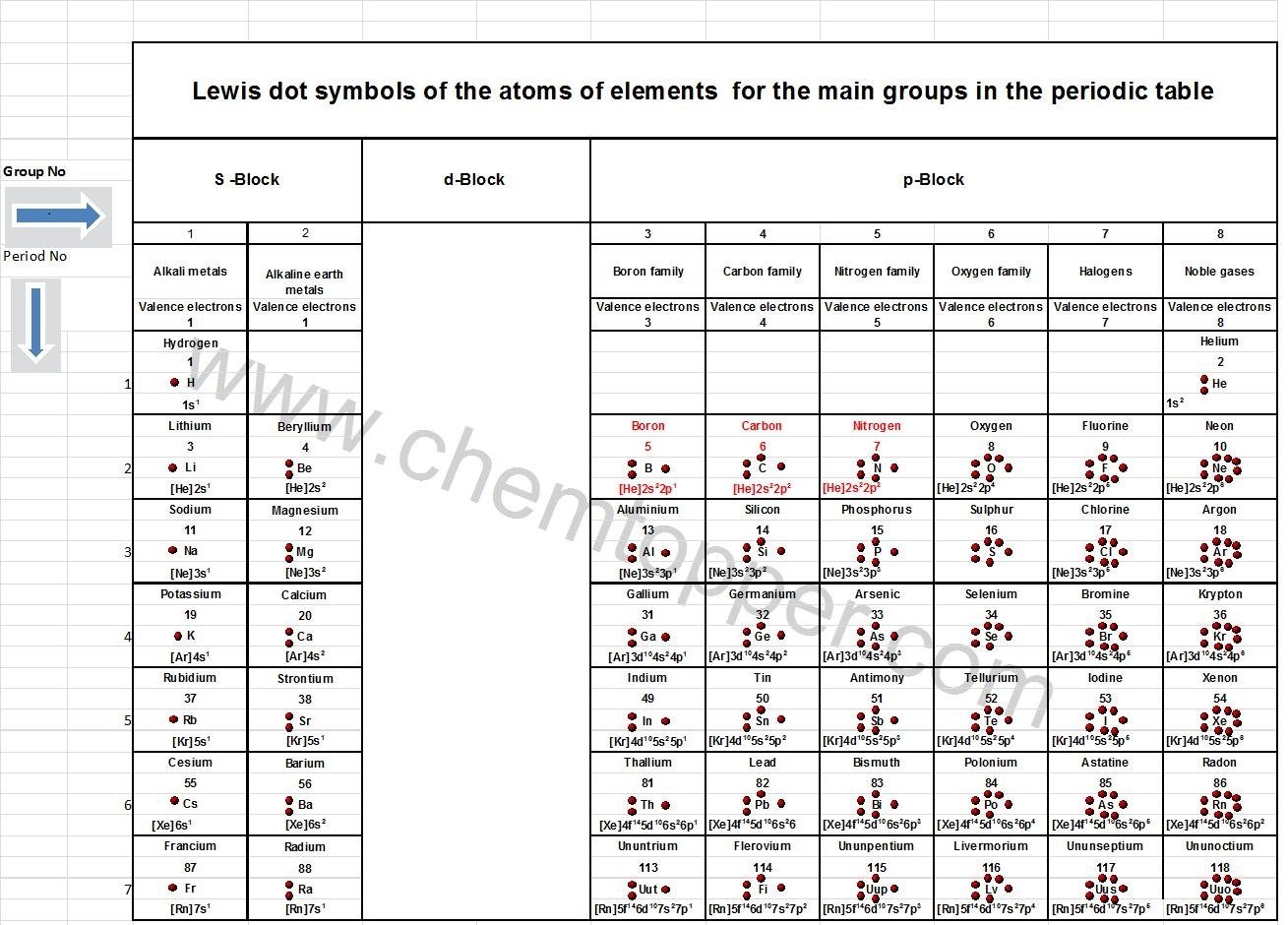
How to Draw Lewis Dot Structure Online Chemistry Tutor

How to draw Lewis Structures a step by step tutorial Middle School

Lewis Dot Structure Definition, Examples, and Drawing

FileInfographic Draw a Lewis Dot Structure. Beaker Babe 2015
Ask Me Questions On Facebook:
The Reason For Learning To Draw Lewis Structures Is To Predict The Number And Type Of Bonds That May Be Formed Around An Atom.
Thus Far, We Have Discussed The Various Types Of Bonds That Form Between Atoms And/Or Ions.
2.1M Views 6 Years Ago New Ap & General Chemistry Video Playlist.
Related Post: