Mixtures Drawing
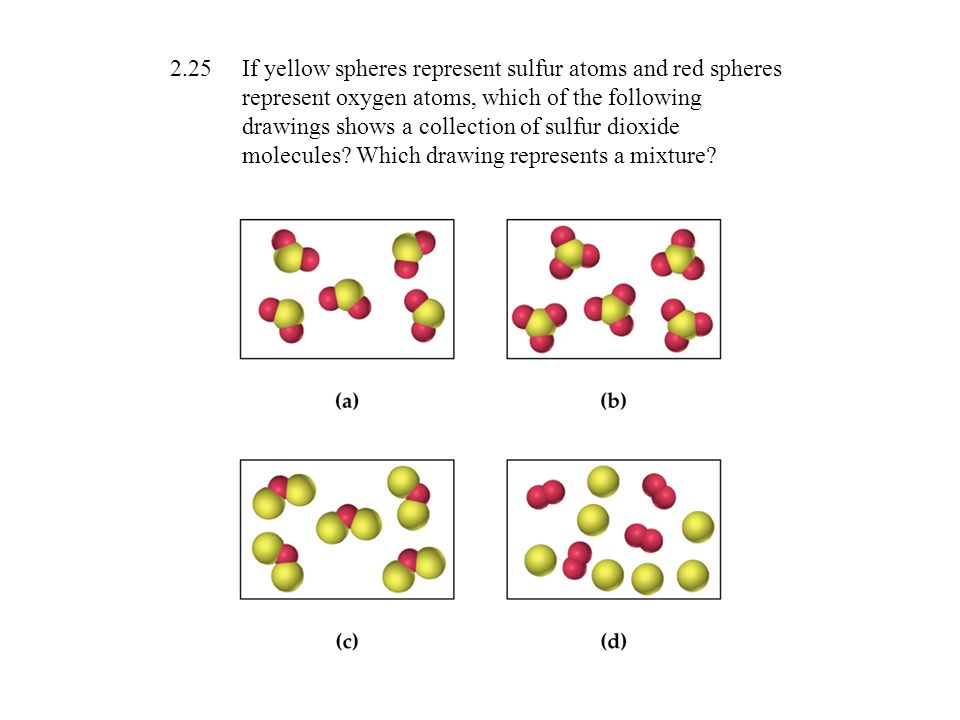
Mixtures Drawing - Web the difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their composition. Here's a closer look at these types of mixtures and examples of mixtures. Each of the layers is called a phase. Distillation takes advantage of differences in boiling points. In the second drawing, we have an excess of right gloves compared to left gloves. Filtration separates solids of different sizes. By the end of this lesson, students should be able to. Consists of two or more different elements and/or compounds physically intermingled, can be separated into its components by physical means, and. The composition of the mixture is the. Web there are two types of mixtures: Homogeneous mixtures and heterogeneous mixtures. By the end of this lesson, students should be able to. This is called a racemic mixture of enantiomers. Web drawing particulate models of reaction mixtures. The most common type of homogenous mixture is a solution, which can be a solid, liquid, or gas. By the end of this lesson, students should be able to. In this video, we'll learn how to represent the relative concentrations of the substances in a solution as well as the interactions between the substances using a particulate model. This animation explores different ways of separating a variety of mixtures. Web there are two types of mixtures: Consists of. In contrast, a homogeneous mixture has a uniform composition. Web last updated december 12, 2023. Heterogeneous mixtures have visually distinguishable components, while homogeneous mixtures appear uniform throughout. Mixtures are physical combinations of two or more elements and/or compounds. Web choose from mixtures drawing stock illustrations from istock. Matter can be classified into two broad categories: In chemistry, a mixture is a combination that does not produce a chemical reaction. Web updated on october 02, 2020. A rock, for example, is a solid. Separate homogeneous from inhomogeneous mixtures. This is called a racemic mixture of enantiomers. Homogeneous mixtures and heterogeneous mixtures. Have students draw the particle models for salt and water separately. Web scientific and engineering practices : A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. Solids are relatively rigid and have fixed shapes and volumes. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. Here's a closer look at these types of mixtures and examples of mixtures. A heterogeneous mixture consists of two or more phases. A given chemical reaction can be represented. A balanced chemical equation can be visualized using a particulate diagram, in which each of the atoms involved in the reaction is represented using a circle or a sphere. This is a quick revision activity to check students' understanding of the different methods used to separate mixtures. No matter where you sample the mixture, the amount and type of components. The separation techniques addressed in this animation include filtration, evaporation, distillation, and chromatography (focusing on paper chromatography). In the first drawing, we have an equal number of left and right gloves (i.e. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. Each of the layers is called a. In the first drawing, we have an equal number of left and right gloves (i.e. Consists of two or more different elements and/or compounds physically intermingled, can be separated into its components by physical means, and. Homogeneous mixtures and heterogeneous mixtures. A balanced chemical equation can be visualized using a particulate diagram, in which each of the atoms involved in. Often retains many of the properties of its components. Web learn about elements, compounds and mixtures in this ks3 chemistry guide from bbc bitesize. Web updated on october 02, 2020. A heterogeneous mixture consists of two or more phases. A solution is a homogeneous mixture composed of two or more pure substances. Classify particle diagrams as representing elements, compounds, or mixtures thereof. Have students draw the particle models for salt and water separately. In contrast, a homogeneous mixture has a uniform composition. Chromatography involves solvent separation on a solid medium. Web by definition, a pure substance or a homogeneous mixture consists of a single phase. Web the difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their composition. Examples include steel, wine, and air. The separation techniques addressed in this animation include filtration, evaporation, distillation, and chromatography (focusing on paper chromatography). A solution is a homogeneous mixture composed of two or more pure substances. Web scientific and engineering practices : By the end of this lesson, students should be able to. Web last updated december 12, 2023. A heterogeneous mixture consists of two or more phases. Separate homogeneous from inhomogeneous mixtures. There are two categories of mixtures: Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water).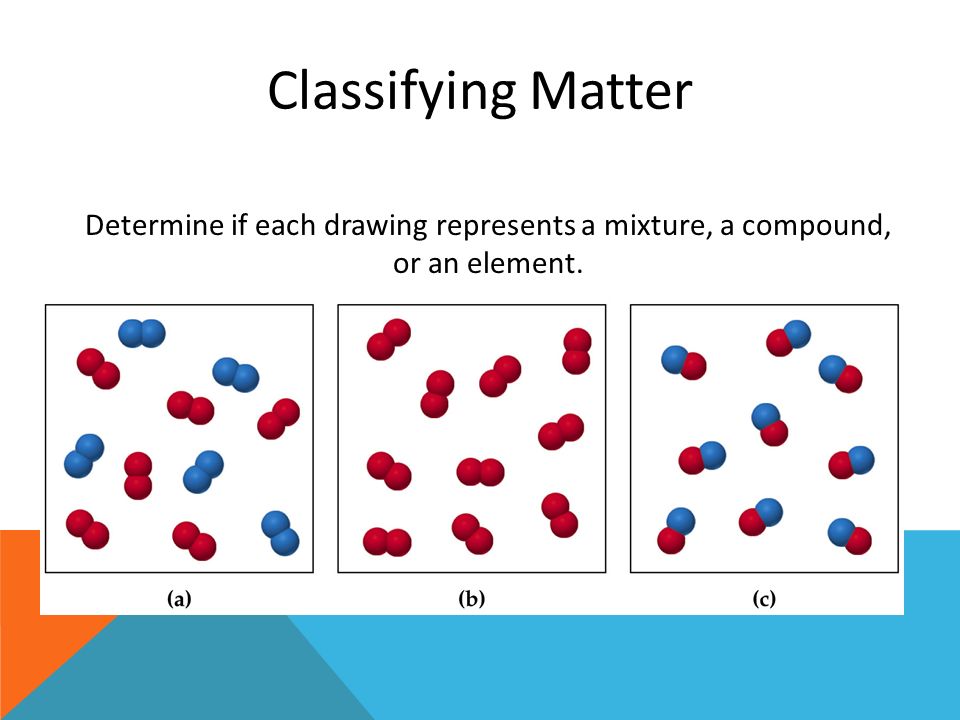
Mixture Drawing at Explore collection of Mixture
Mixture Drawing at Explore collection of Mixture

What Is a Heterogeneous Mixture? Definition and Examples
:max_bytes(150000):strip_icc()/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)
10 Heterogeneous and Homogeneous Mixtures

Homogeneous Vs Heterogenous Mixtures MyWaterEarth&Sky
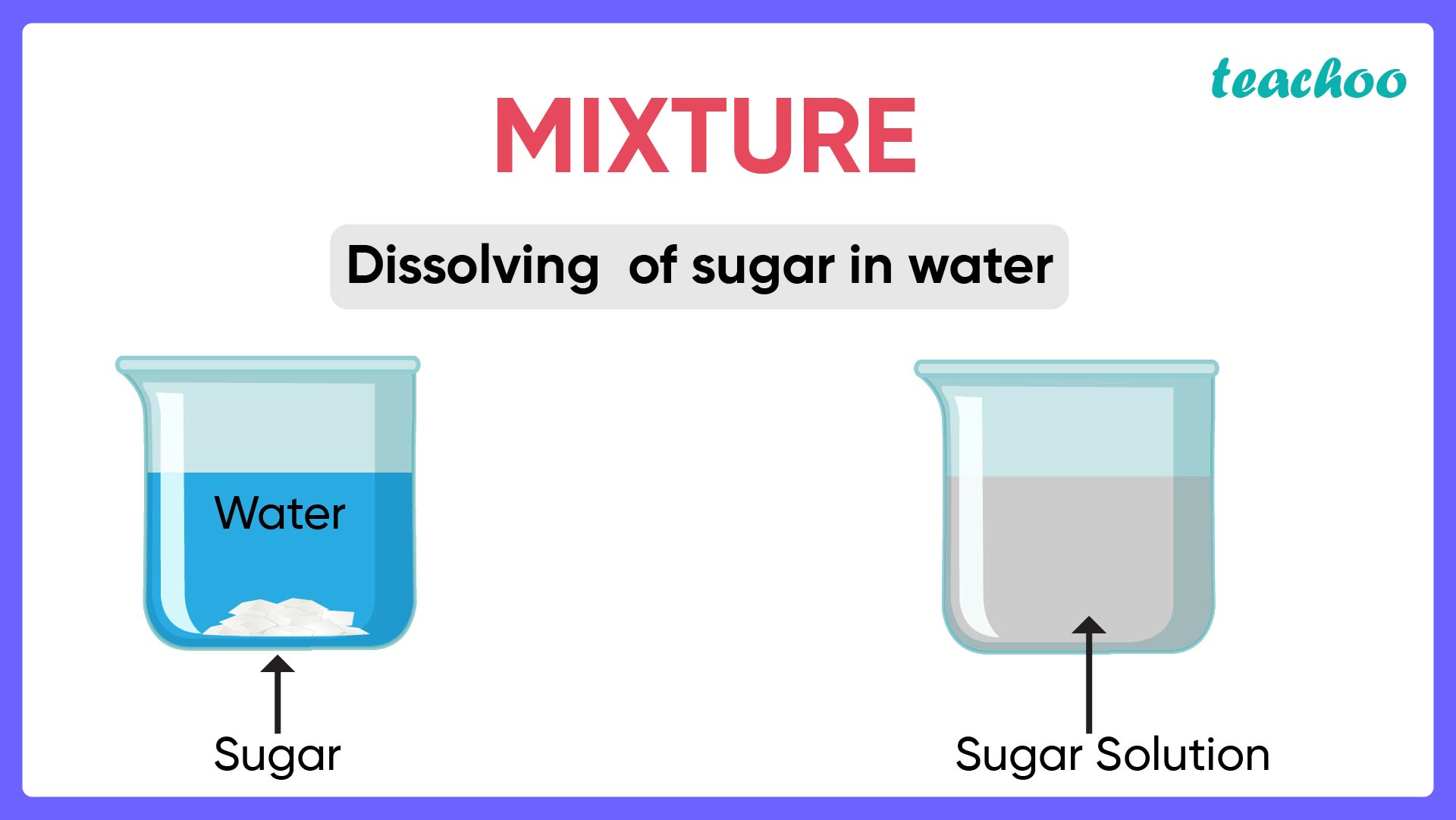
What is a Mixture? Types of Mixtures Chemistry Teachoo
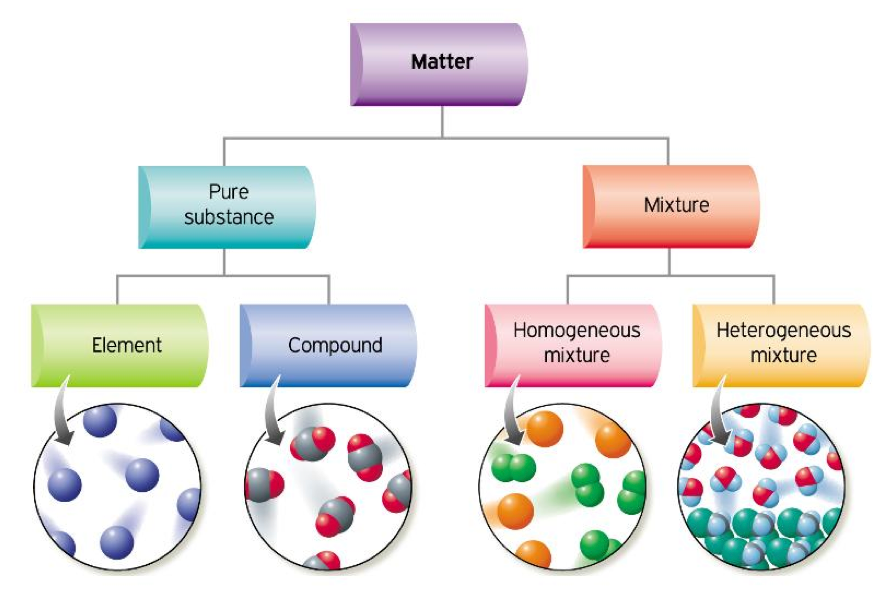
Properties of Mixtures ACA Grade 8 Science

10 Examples of Mixtures
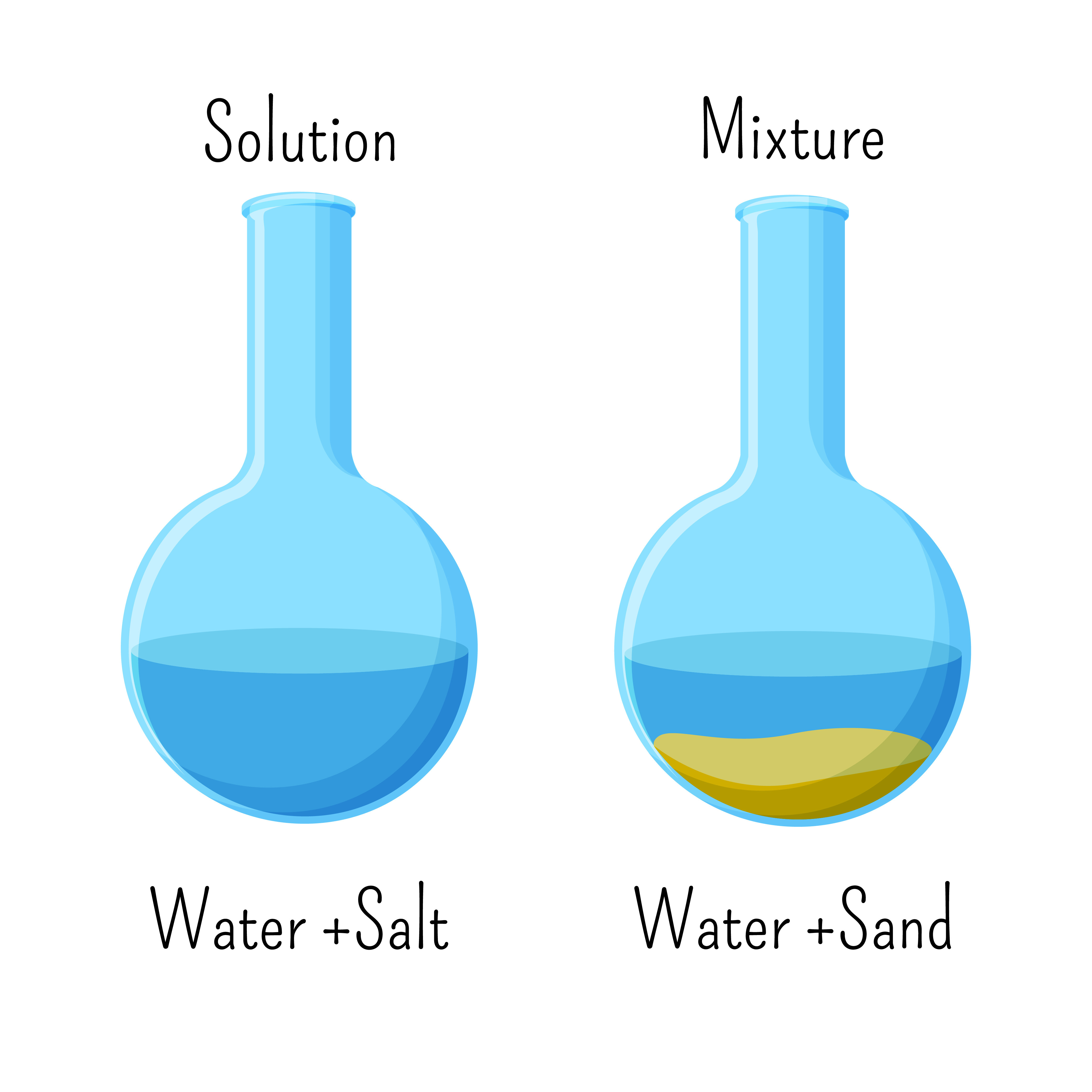
Chemistry Solutions And Mixtures Level 1 activity for kids
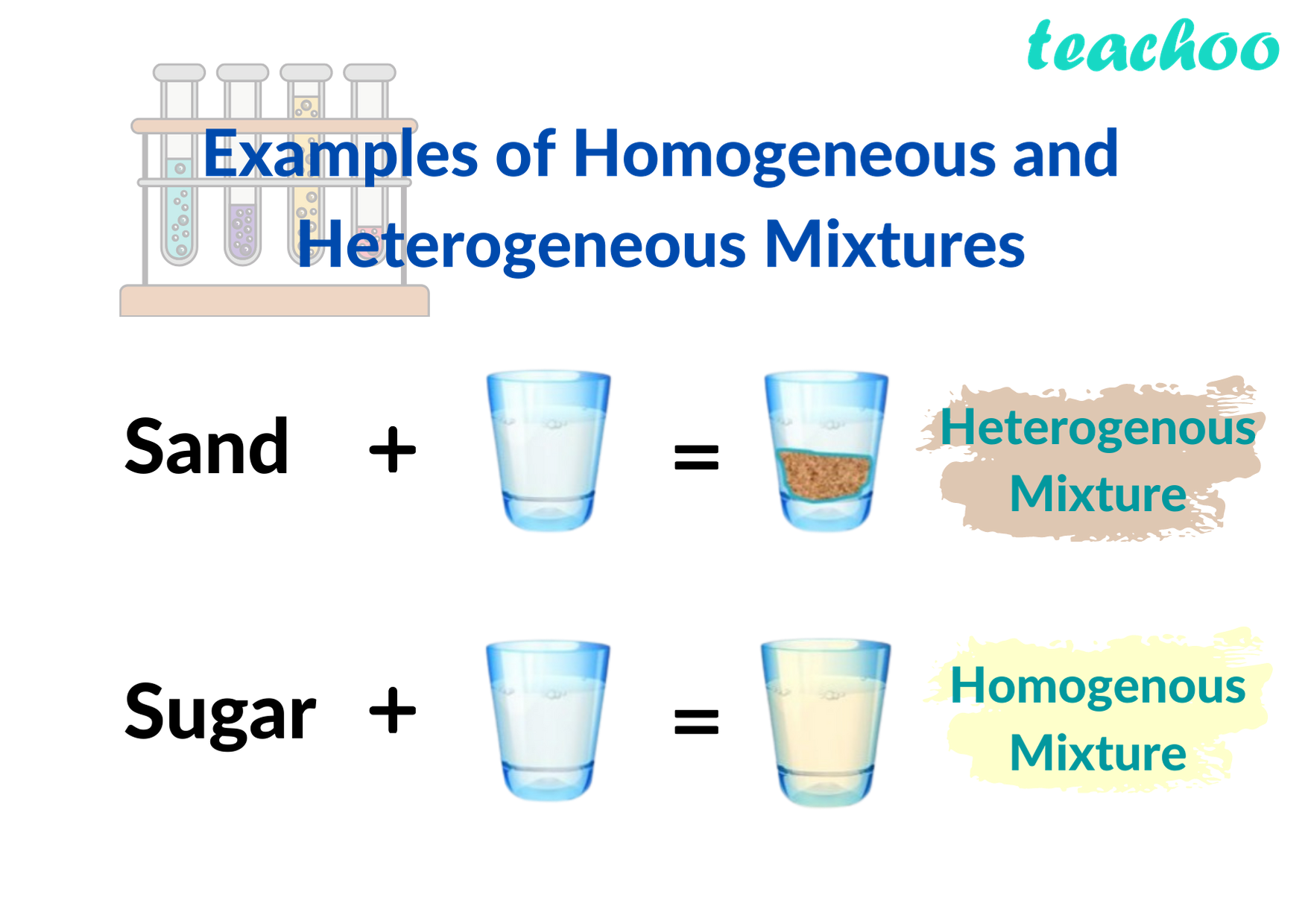
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo
Web Mixtures Can Be Separated Using A Variety Of Techniques.
A Given Chemical Reaction Can Be Represented Using A Particulate Diagram, In Which The Reaction Mixture Is Depicted Both Before The Reaction Occurs And After The Reaction Has Proceeded Completely As Possible.
Define Terms Related To Pure Substances And Mixtures.
Here's A Closer Look At These Types Of Mixtures And Examples Of Mixtures.
Related Post: