Nacl Drawing
Nacl Drawing - Both ions show octahedral coordination (cn = 6). Details about it are given below. Learn what is it and how to draw it for nacl. It occurs in oceans and sea waters. Laboratory chemical safety summary (lcss) datasheet. It is also found as rock salt. Rock salt (nacl) other unit cell types. Web learn how to draw the structure of sodium chloride in a simple and easy way with this video tutorial. Web the structure of nacl is formed by repeating the face centered cubic unit cell. Web what is sodium chloride? The salient features of its structure are: Count the nacl lewis structure valence electrons by adding the valence electrons of both sodium and chlorine atoms. All octahedral holes in a cubic close packing are occupied by counterions. The different structure of cesium chloride; Click on the images below to view the nacl lattice structure rotating. Rock salt (nacl) other unit cell types. Learn what is it and how to draw it for nacl. 59k views 3 years ago. ∴ total available valence electrons = 1 + 7 = 8 Web sodium chloride , also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and. Count the nacl lewis structure valence electrons by adding the valence electrons of both sodium and chlorine atoms. For nacl we have an ionic compound and we need to take that. Why are the cesium chloride and sodium chloride structures different? Nacl 6 and clna 6 octahedra. A given chemical reaction can be represented using a particulate diagram, in which. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. Get all your chemistry doubts resolved by enthuziastic expert teachers. How to draw this structure; Sodium chloride is an ionic compound with the chemical formula nacl. Count the available valence electrons. Since they’re from opposite sides of the periodic table, they form an ionic compound. All octahedral holes in a cubic close packing are occupied by counterions. It occurs in oceans and sea waters. ∴ total available valence electrons = 1 + 7 = 8 How to draw this structure; Web sodium chloride / ˌsoʊdiəm ˈklɔːraɪd /, [8] commonly known as edible salt, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chlorine ions. It has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. Computed by pubchem 2.2 (pubchem release 2021.10.14) component compounds. Web learn how to draw the. Valence shell electrons prediction of molecule in the form of diagram with lines showing and dots showing electrons are lewis structure. In this i have shown how to draw structure of sodium chloride (nacl). It occurs in oceans and sea waters. The cell looks the same whether you start with anions or cations on the corners. Web the structure of. It simply describes how to draw the structure of a sodium chloride lattice. Sodium chloride is also known as salt. A given chemical reaction can be represented using a particulate diagram, in which the reaction mixture is depicted both before the reaction occurs and after the reaction has proceeded completely as possible. Sodium chloride has a high melting and boiling. Sodium chloride is also known as salt. The salient features of its structure are: How to draw this structure; Rock salt (nacl) other unit cell types. Web check me out: How to draw a simple sodium chloride lattice. This is the diagram we are aiming at: In its aqueous form, it is called a saline solution. The different structure of cesium chloride; The cell looks the same whether you start with anions or cations on the corners. For nacl we have an ionic compound and we need to take that. The salient features of its structure are: Sodium chloride has a high melting and boiling point ∴ total available valence electrons = 1 + 7 = 8 In the periodic table, sodium is in the first group and chlorine is in the 17th. This is the diagram we are aiming at: Count the nacl lewis structure valence electrons by adding the valence electrons of both sodium and chlorine atoms. A given chemical reaction can be represented using a particulate diagram, in which the reaction mixture is depicted both before the reaction occurs and after the reaction has proceeded completely as possible. It is a crystalline solid, white. 59k views 3 years ago. It occurs in oceans and sea waters. 24k views 3 years ago lewis structures. Here is how to draw the crystal structure of. Nacl 6 and clna 6 octahedra. Web nacl has a cubic unit cell. The cell looks the same whether you start with anions or cations on the corners.
Chemistry model salt molecule diatomic sodium chlorine NaCl scientific

How To Draw Structure Of Sodium Chloride Nacl Youtube Images and

Kristallstruktur Nacl Chemische Kostenlose auf Pixabay

Structure of Sodium Chloride (NaCl) Formation of NaCl How to draw

Structure of NaCl (Sodium chloride) YouTube

subatomic particles Montessori Muddle

Class1112th। How to draw Nacl crystal structure? Nacl क्रिस्टल संरचना

Nacl structure,nacl crystal structure,how to draw nacl structure

Sodium Chloride Nacl Molecular Cube HighRes Vector Graphic Getty Images
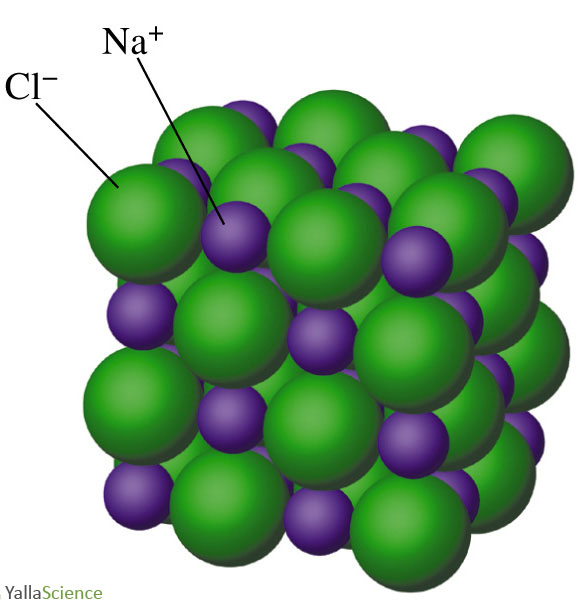
Formula unit of NaCl, sodium chloride Chemistry Dictionary
Web Sodium Chloride / ˌSoʊdiəm ˈKlɔːraɪd /, [8] Commonly Known As Edible Salt, Is An Ionic Compound With The Chemical Formula Nacl, Representing A 1:1 Ratio Of Sodium And Chlorine Ions.
283K Views 5 Years Ago United States.
Sodium Chloride Is An Ionic Compound With The Chemical Formula Nacl.
Valence Shell Electrons Prediction Of Molecule In The Form Of Diagram With Lines Showing And Dots Showing Electrons Are Lewis Structure.
Related Post: