Universal Solvent Drawing
Universal Solvent Drawing - Water fountains work because of water's. Explore the history and types of solvents, and the concept of alkahest, a hypothetical. This is, of course, another key property of water because more substances dissolve in water than any other common liquid. Web anne marie helmenstine, ph.d. Web learn how water's polar molecules enhance its dissolving power and break down salts into ions. A solvent is a substance that dissolves, or breaks apart, another substance (known as a solute). Web learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density make it essential for life on earth. Water is capable of dissolving a variety of. Web are nonpolar water 'loving' molecules. Water sticks to pine needles due to adhesion; Web learn how water's polar molecules enhance its dissolving power and break down salts into ions. Of course it cannot dissolve everything, but it does dissolve more. Explore the history and types of solvents, and the concept of alkahest, a hypothetical. See a drawing of salt (nacl) dissolving in water and the role of water molecules. Water sticks to pine. Web because of its high polarity, water is called the universal solvent. Water sticks to pine needles due to adhesion; Water is known as the universal solvent. See diagrams and examples of. Explore the history and types of solvents, and the concept of alkahest, a hypothetical. Water is known as the universal solvent. This is because the polar. Water is sometimes referred to as the “universal solvent,” because it dissolves more compounds than any other liquid known. Web and, water is called the universal solvent because it dissolves more substances than any other liquid. Web learn how water's polar molecules enhance its dissolving power and break. Water sticks to pine needles due to adhesion; Web learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density make it essential for life on earth. Web anne marie helmenstine, ph.d. Salt crystals, halocline eggs, eggshell. Water is known as the universal solvent. Web water is commonly referred to as the universal solvent, but what does that mean? Water is known as the universal solvent. Web water, the universal solvent water molecules clump together due to cohesion; Of course it cannot dissolve everything, but it does dissolve more. Web learn why water is the universal solvent and how its polarity, surface tension, specific. Web learn what a universal solvent is and why water is often called the universal solvent. Web learn why water is the universal solvent and how its polarity, surface tension, specific heat, and density make it essential for life on earth. A solvent is a substance that dissolves, or breaks apart, another substance (known as a solute). It’s not actually. See a drawing of salt (nacl) dissolving in water and the role of water molecules. Web learn what a universal solvent is and why water is often called the universal solvent. Web anne marie helmenstine, ph.d. Web learn how water's polar molecules enhance its dissolving power and break down salts into ions. Here is an explanation of why water is. Web learn how water's polar molecules enhance its dissolving power and break down salts into ions. See a drawing of salt (nacl) dissolving in water and the role of water molecules. See diagrams and examples of. Water is capable of dissolving a variety of. Water is known as the universal solvent. Here is an explanation of why water is called the universal solvent. Web and, water is called the universal solvent because it dissolves more substances than any other liquid. Water fountains work because of water's. Web water is commonly referred to as the universal solvent, but what does that mean? This is, of course, another key property of water because. Web are nonpolar water 'loving' molecules. This is because the polar. A solution is formed when one substance dissolves in another. Web water is commonly referred to as the universal solvent, but what does that mean? Web learn how water's polar molecules enhance its dissolving power and break down salts into ions. We need to take the statement water is the universal solvent with a grain of salt (pun intended). Web are nonpolar water 'loving' molecules. Water is capable of dissolving a variety of. Water is known as the universal solvent. Web anne marie helmenstine, ph.d. Water is sometimes referred to as the “universal solvent,” because it dissolves more compounds than any other liquid known. Water sticks to pine needles due to adhesion; Web learn what a universal solvent is and why water is often called the universal solvent. Web water is commonly referred to as the universal solvent, but what does that mean? Of course it cannot dissolve everything, but it does dissolve more. Updated on september 09, 2019. A solution is formed when one substance dissolves in another. Here is an explanation of why water is called the universal solvent. Web and, water is called the universal solvent because it dissolves more substances than any other liquid. See diagrams and examples of. This is important to every living thing on earth.
PPT 3/27 Notes on Mixtures PowerPoint Presentation, free download
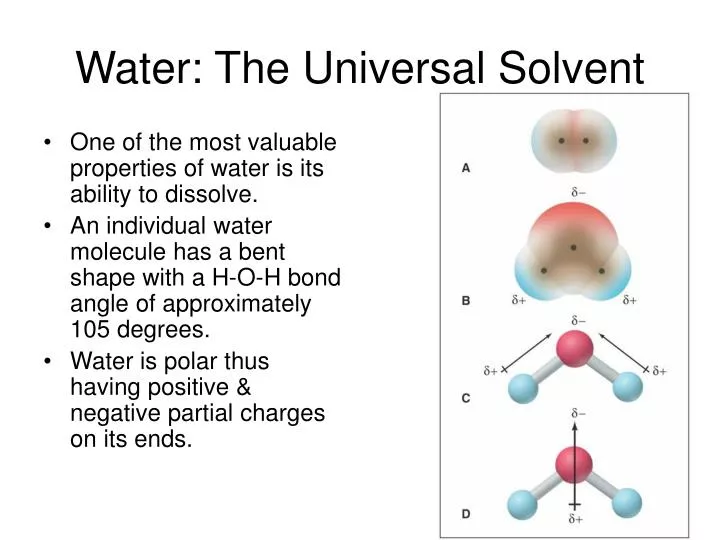
PPT Water The Universal Solvent PowerPoint Presentation, free

PPT Properties of Solutions PowerPoint Presentation, free download

6.3 Classifying Chemical Reactions General College Chemistry I

PPT Chemical And Physical Features of Seawater PowerPoint
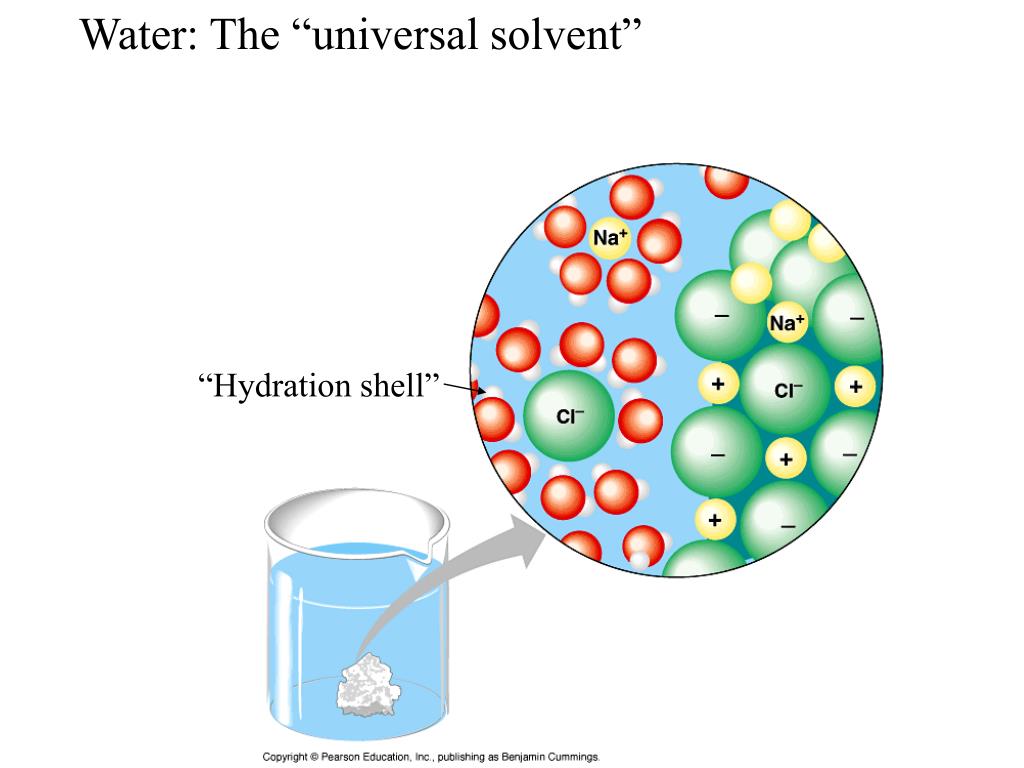
What Is A Solvent 1 5 What Are Solutions The tears in your eyes are
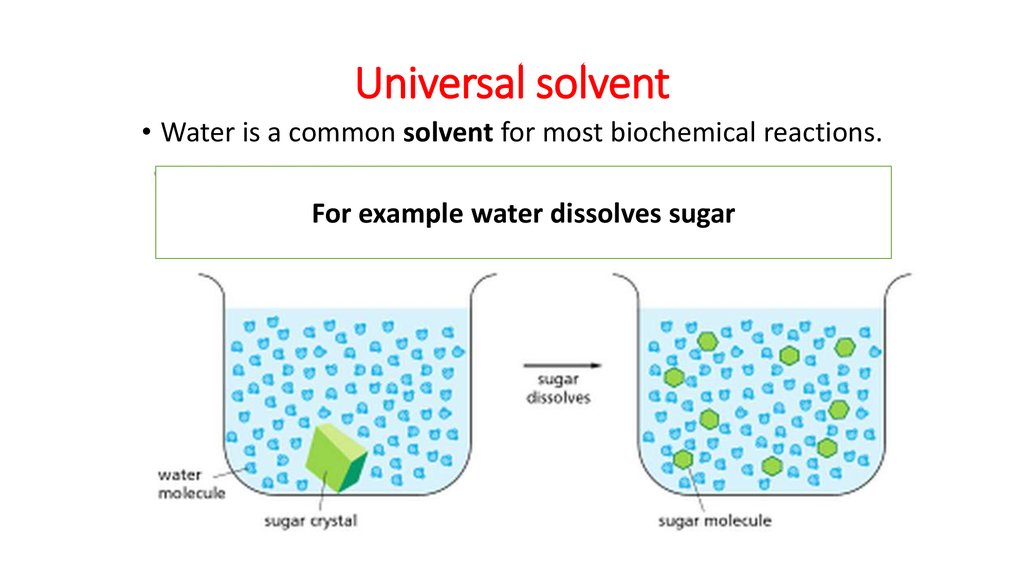
Class water online presentation
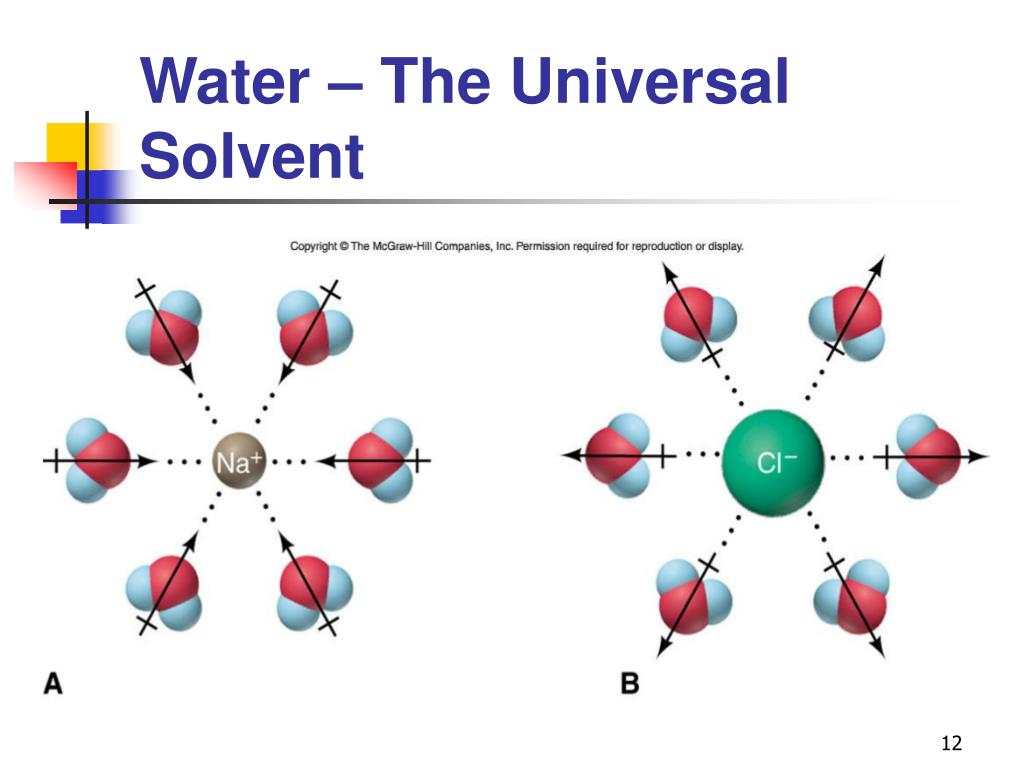
PPT Chapter 7 PowerPoint Presentation, free download ID6009487

PPT Unit 7 Solution Chemistry Chapter 13 PowerPoint Presentation
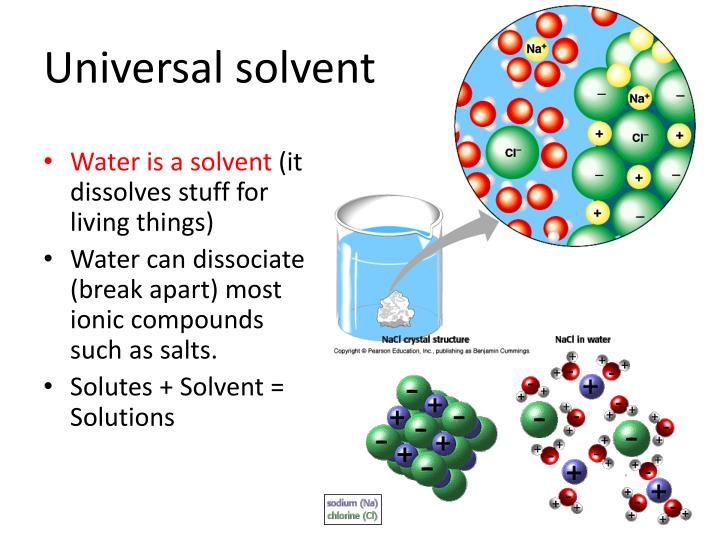
PPT Properties of Water PowerPoint Presentation ID2370697
This Is Because The Polar.
Water Fountains Work Because Of Water's.
Web Because Of Its High Polarity, Water Is Called The Universal Solvent.
With 70% Of Our Earth Being Ocean Water And 65% Of Our Bodies Being Water, It Is Hard To Not Be Aware Of How Important It Is In Our.
Related Post: